Alveolar macrophages support interferon gamma-mediated viral clearance in RSV-infected neonatal mice
- PMID: 26438053
- PMCID: PMC4594958
- DOI: 10.1186/s12931-015-0282-7
Alveolar macrophages support interferon gamma-mediated viral clearance in RSV-infected neonatal mice
Abstract
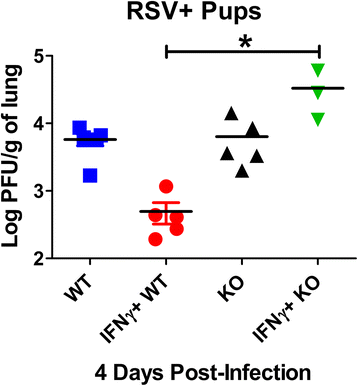
Background: Poor interferon gamma (IFNγ) production during respiratory syncytial virus (RSV) is associated with prolonged viral clearance and increased disease severity in neonatal mice and humans. We previously showed that intra-nasal delivery of IFNγ significantly enhances RSV clearance from neonatal lungs prior to observed T-lymphocyte recruitment or activation, suggesting an innate immune mechanism of viral clearance. We further showed that alveolar macrophages dominate the RSV-infected neonatal airways relative to adults, consistent with human neonatal autopsy data. Therefore, the goal of this work was to determine the role of neonatal alveolar macrophages in IFNγ-mediated RSV clearance.
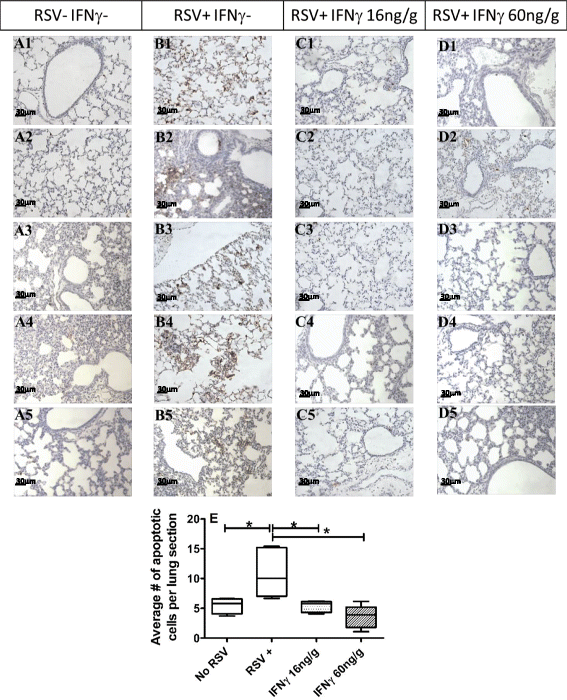
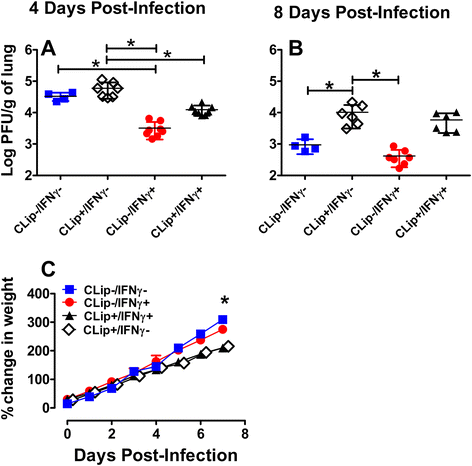
Methods: Clodronate liposomes, flow cytometry, viral plaque assays, and histology were used to examine the role of alveolar macrophages (AMs) and the effects of intra-nasal IFNγ in RSV infected neonatal Balb/c mice. The functional outcomes of AM depletion were determined quantitatively by viral titers using plaque assay. Illness was assessed by measuring reduced weight gain.
Results: AM activation during RSV infection was age-dependent and correlated tightly with IFNγ exposure. Higher doses of IFNγ more efficiently stimulated AM activation and expedited RSV clearance without significantly affecting weight gain. The presence of AMs were independently associated with improved RSV clearance, whereas AM depletion but not IFNγ exposure, significantly impaired weight gain in RSV-infected neonates.
Conclusion: We show here for the first time, that IFNγ is critical for neonatal RSV clearance and that it depends, in part, on alveolar macrophages (AMs) for efficient viral clearing effects. Early reductions in viral burden are likely to have profound short- and long-term immune effects in the vulnerable post-natally developing lung environment. Studies are ongoing to elucidate the pathologic effects associated with early versus delayed RSV clearance in developing neonatal airways.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

