A Nonsense Variant in COL6A1 in Landseer Dogs with Muscular Dystrophy
- PMID: 26438297
- PMCID: PMC4683634
- DOI: 10.1534/g3.115.021923
A Nonsense Variant in COL6A1 in Landseer Dogs with Muscular Dystrophy
Abstract
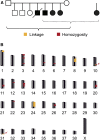
A novel canine muscular dystrophy in Landseer dogs was observed. We had access to five affected dogs from two litters. The clinical signs started at a few weeks of age, and the severe progressive muscle weakness led to euthanasia between 5 and 15 months of age. The pedigrees of the affected dogs suggested a monogenic autosomal-recessive inheritance of the trait. Linkage and homozygosity mapping indicated two potential genome segments for the causative variant on chromosomes 10 and 31 harboring a total of 4.8 Mb of DNA or 0.2% of the canine genome. Using the Illumina sequencing technology, we obtained a whole-genome sequence from one affected Landseer. Variants were called with respect to the dog reference genome and compared with the genetic variants of 170 control dogs from other breeds. The affected Landseer dog was homozygous for a single, private nonsynonymous variant in the critical intervals, a nonsense variant in the COL6A1 gene (Chr31:39,303,964G>T; COL6A1:c.289G>T; p.E97*). Genotypes at this variant showed perfect concordance with the muscular dystrophy phenotype in all five cases and more than 1000 control dogs. Variants in the human COL6A1 gene cause Bethlem myopathy or Ullrich congenital muscular dystrophy. We therefore conclude that the identified canine COL6A1 variant is most likely causative for the observed muscular dystrophy in Landseer dogs. On the basis of the nature of the genetic variant in Landseer dogs and their severe clinical phenotype these dogs represent a model for human Ullrich congenital muscular dystrophy.
Keywords: Mendelian traits; animal model; dog; veterinary genetics; whole-genome sequencing.
Copyright © 2015 Steffen et al.
Figures


References
-
- Abecasis G. R., Cherny S. S., Cookson W. O., Cardon L. R., 2002. Merlin—rapid analysis of dense genetic maps using sparse gene flow trees. Nat. Genet. 30: 97–101. - PubMed
-
- Bonaldo P., Braghetta P., Zanetti M., Piccolo S., Volpin D., et al. , 1998. Collagen VI deficiency induces early onset myopathy in the mouse: an animal model for Bethlem myopathy. Hum. Mol. Genet. 7: 2135–2140. - PubMed
-
- Briñas L. P., Richard S., Quijano-Roy C., Gartioux C., Ledeuil C., et al. , 2010. Early onset collagen VI myopathies: genetic and clinical correlations. Ann. Neurol. 68: 511–520. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
