Brucella canis is an intracellular pathogen that induces a lower proinflammatory response than smooth zoonotic counterparts
- PMID: 26438796
- PMCID: PMC4645416
- DOI: 10.1128/IAI.00995-15
Brucella canis is an intracellular pathogen that induces a lower proinflammatory response than smooth zoonotic counterparts
Abstract
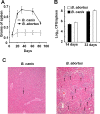
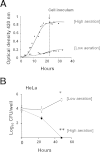
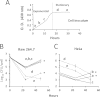
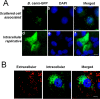
Canine brucellosis caused by Brucella canis is a disease of dogs and a zoonotic risk. B. canis harbors most of the virulence determinants defined for the genus, but its pathogenic strategy remains unclear since it has not been demonstrated that this natural rough bacterium is an intracellular pathogen. Studies of B. canis outbreaks in kennel facilities indicated that infected dogs displaying clinical signs did not present hematological alterations. A virulent B. canis strain isolated from those outbreaks readily replicated in different organs of mice for a protracted period. However, the levels of tumor necrosis factor alpha, interleukin-6 (IL-6), and IL-12 in serum were close to background levels. Furthermore, B. canis induced lower levels of gamma interferon, less inflammation of the spleen, and a reduced number of granulomas in the liver in mice than did B. abortus. When the interaction of B. canis with cells was studied ex vivo, two patterns were observed, a predominant scattered cell-associated pattern of nonviable bacteria and an infrequent intracellular replicative pattern of viable bacteria in a perinuclear location. The second pattern, responsible for the increase in intracellular multiplication, was dependent on the type IV secretion system VirB and was seen only if the inoculum used for cell infections was in early exponential phase. Intracellular replicative B. canis followed an intracellular trafficking route undistinguishable from that of B. abortus. Although B. canis induces a lower proinflammatory response and has a stealthier replication cycle, it still displays the pathogenic properties of the genus and the ability to persist in infected organs based on the ability to multiply intracellularly.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures







References
-
- Barquero-Calvo E, Chaves-Olarte E, Weiss DS, Guzmán-Verri C, Chacón-Díaz C, Rucavado A, Moriyón I, Moreno E. 2007. Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of the infection. PLoS One 2:e631. doi:10.1371/journal.pone.0000631. - DOI - PMC - PubMed
-
- Moreno E, Moriyón I. 2006. The genus Brucella, p 315–456. In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrant E (ed), The prokaryotes, vol 5 Springer Verlag, New York, NY.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

