p21-activated Kinases (PAKs) Mediate the Phosphorylation of PREX2 Protein to Initiate Feedback Inhibition of Rac1 GTPase
- PMID: 26438819
- PMCID: PMC4661405
- DOI: 10.1074/jbc.M115.668244
p21-activated Kinases (PAKs) Mediate the Phosphorylation of PREX2 Protein to Initiate Feedback Inhibition of Rac1 GTPase
Abstract
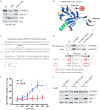
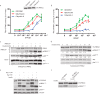
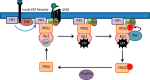
Phosphatidylinositol 3,4,5-trisphosphate (PIP3)-dependent Rac exchanger 2 (PREX2) is a guanine nucleotide exchange factor (GEF) for the Ras-related C3 botulinum toxin substrate 1 (Rac1) GTPase, facilitating the exchange of GDP for GTP on Rac1. GTP-bound Rac1 then activates its downstream effectors, including p21-activated kinases (PAKs). PREX2 and Rac1 are frequently mutated in cancer and have key roles within the insulin-signaling pathway. Rac1 can be inactivated by multiple mechanisms; however, negative regulation by insulin is not well understood. Here, we show that in response to being activated after insulin stimulation, Rac1 initiates its own inactivation by decreasing PREX2 GEF activity. Following PREX2-mediated activation of Rac1 by the second messengers PIP3 or Gβγ, we found that PREX2 was phosphorylated through a PAK-dependent mechanism. PAK-mediated phosphorylation of PREX2 reduced GEF activity toward Rac1 by inhibiting PREX2 binding to PIP3 and Gβγ. Cell fractionation experiments also revealed that phosphorylation prevented PREX2 from localizing to the cellular membrane. Furthermore, the onset of insulin-induced phosphorylation of PREX2 was delayed compared with AKT. Altogether, we propose that second messengers activate the Rac1 signal, which sets in motion a cascade whereby PAKs phosphorylate and negatively regulate PREX2 to decrease Rac1 activation. This type of regulation would allow for transient activation of the PREX2-Rac1 signal and may be relevant in multiple physiological processes, including diseases such as diabetes and cancer when insulin signaling is chronically activated.
Keywords: G-protein-coupled receptor (GPCR); Phosphatidylinositol-3,4,5-trisphosphate-dependent RAC exchanger 2 (PREX2); Rac (Rac GTPase); guanine nucleotide exchange factor (GEF); insulin; phosphatidylinositide 3-kinase (PI 3-kinase); serine/threonine-protein kinase PAK 1 (PAK1); signal transduction.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Donald S., Hill K., Lecureuil C., Barnouin R., Krugmann S., John Coadwell W., Andrews S. R., Walker S. A., Hawkins P. T., Stephens L. R., and Welch H. C. (2004) P-Rex2, a new guanine-nucleotide exchange factor for Rac. FEBS Lett. 572, 172–176 - PubMed
-
- Rosenfeldt H., Vázquez-Prado J., and Gutkind J. S. (2004) P-REX2, a novel PI-3-kinase sensitive Rac exchange factor. FEBS Lett. 572, 167–171 - PubMed
-
- Cherfils J., and Zeghouf M. (2013) Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 93, 269–309 - PubMed
-
- Abo A., Pick E., Hall A., Totty N., Teahan C. G., and Segal A. W. (1991) Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 353, 668–670 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

