Macrophage-specific de Novo Synthesis of Ceramide Is Dispensable for Inflammasome-driven Inflammation and Insulin Resistance in Obesity
- PMID: 26438821
- PMCID: PMC4705943
- DOI: 10.1074/jbc.M115.680199
Macrophage-specific de Novo Synthesis of Ceramide Is Dispensable for Inflammasome-driven Inflammation and Insulin Resistance in Obesity
Abstract
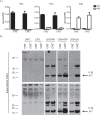
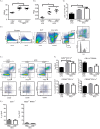
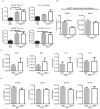
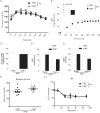
Dietary lipid overload and calorie excess during obesity is a low grade chronic inflammatory state with diminished ability to appropriately metabolize glucose or lipids. Macrophages are critical in maintaining adipose tissue homeostasis, in part by regulating lipid metabolism, energy homeostasis, and tissue remodeling. During high fat diet-induced obesity, macrophages are activated by lipid derived "danger signals" such as ceramides and palmitate and promote the adipose tissue inflammation in an Nlrp3 inflammasome-dependent manner. Given that the metabolic fate of fatty acids in macrophages is not entirely elucidated, we have hypothesized that de novo synthesis of ceramide, through the rate-limiting enzyme serine palmitoyltransferase long chain (Sptlc)-2, is required for saturated fatty acid-driven Nlrp3 inflammasome activation in macrophages. Here we report that mitochondrial targeted overexpression of catalase, which is established to mitigate oxidative stress, controls ceramide-induced Nlrp3 inflammasome activation but does not affect the ATP-mediated caspase-1 cleavage. Surprisingly, myeloid cell-specific deletion of Sptlc2 is not required for palmitate-driven Nlrp3 inflammasome activation. Furthermore, the ablation of Sptlc2 in macrophages did not impact macrophage polarization or obesity-induced adipose tissue leukocytosis. Consistent with these data, investigation of insulin resistance using hyperinsulinemic-euglycemic clamps revealed no significant differences in obese mice lacking ceramide de novo synthesis machinery in macrophages. These data suggest that alternate metabolic pathways control fatty acid-derived ceramide synthesis in macrophage and the Nlrp3 inflammasome activation in obesity.
Keywords: adipose tissue; ceramide synthesis; inflammasome; insulin resistance; macrophage; obesity; saturated fat.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Hossain P., Kawar B., and El Nahas M. (2007) Obesity and diabetes in the developing world: a growing challenge. N. Engl. J. Med. 356, 213–215 - PubMed
-
- Gregor M. F., and Hotamisligil G. S. (2011) Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445 - PubMed
-
- McNelis J. C., and Olefsky J. M. (2014) Macrophages, immunity, and metabolic disease. Immunity 41, 36–48 - PubMed
-
- Nishimura S., Manabe I., Nagasaki M., Eto K., Yamashita H., Ohsugi M., Otsu M., Hara K., Ueki K., Sugiura S., Yoshimura K., Kadowaki T., and Nagai R. (2009) CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 NS077723/NS/NINDS NIH HHS/United States
- R01 DK040936/DK/NIDDK NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- NS077723/NS/NINDS NIH HHS/United States
- R01 AI105097/AI/NIAID NIH HHS/United States
- R01 AG047632/AG/NIA NIH HHS/United States
- R01 DK090556/DK/NIDDK NIH HHS/United States
- AI105097/AI/NIAID NIH HHS/United States
- AG047632/AG/NIA NIH HHS/United States
- R01 AG043608/AG/NIA NIH HHS/United States
- DK-45735/DK/NIDDK NIH HHS/United States
- DK-40936/DK/NIDDK NIH HHS/United States
- R56 AI105097/AI/NIAID NIH HHS/United States
- DK090556/DK/NIDDK NIH HHS/United States
- AG043608/AG/NIA NIH HHS/United States
- U24 DK059635/DK/NIDDK NIH HHS/United States
- DK-059635/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

