Structure and conformational states of the bovine mitochondrial ATP synthase by cryo-EM
- PMID: 26439008
- PMCID: PMC4718723
- DOI: 10.7554/eLife.10180
Structure and conformational states of the bovine mitochondrial ATP synthase by cryo-EM
Abstract
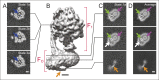
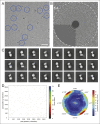
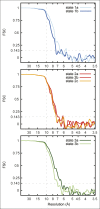
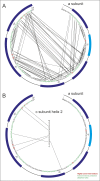
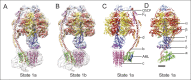
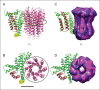
Adenosine triphosphate (ATP), the chemical energy currency of biology, is synthesized in eukaryotic cells primarily by the mitochondrial ATP synthase. ATP synthases operate by a rotary catalytic mechanism where proton translocation through the membrane-inserted FO region is coupled to ATP synthesis in the catalytic F1 region via rotation of a central rotor subcomplex. We report here single particle electron cryomicroscopy (cryo-EM) analysis of the bovine mitochondrial ATP synthase. Combining cryo-EM data with bioinformatic analysis allowed us to determine the fold of the a subunit, suggesting a proton translocation path through the FO region that involves both the a and b subunits. 3D classification of images revealed seven distinct states of the enzyme that show different modes of bending and twisting in the intact ATP synthase. Rotational fluctuations of the c8-ring within the FO region support a Brownian ratchet mechanism for proton-translocation-driven rotation in ATP synthases.
Keywords: ATP synthase; biochemistry; biophysics; bovine; coevolution; cryo-EM; evolutionary covariance; structural biology; structure.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures



References
-
- Baker LA, Watt IN, Runswick MJ, Walker JE, Rubinstein JL. Arrangement of subunits in intact mammalian mitochondrial ATP synthase determined by cryo-EM. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:11675–11680. doi: 10.1073/pnas.1204935109. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

