A Generative Statistical Algorithm for Automatic Detection of Complex Postures
- PMID: 26439258
- PMCID: PMC4595081
- DOI: 10.1371/journal.pcbi.1004517
A Generative Statistical Algorithm for Automatic Detection of Complex Postures
Abstract
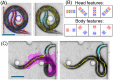
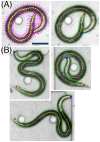
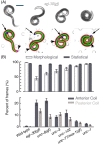
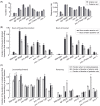
This paper presents a method for automated detection of complex (non-self-avoiding) postures of the nematode Caenorhabditis elegans and its application to analyses of locomotion defects. Our approach is based on progressively detailed statistical models that enable detection of the head and the body even in cases of severe coilers, where data from traditional trackers is limited. We restrict the input available to the algorithm to a single digitized frame, such that manual initialization is not required and the detection problem becomes embarrassingly parallel. Consequently, the proposed algorithm does not propagate detection errors and naturally integrates in a "big data" workflow used for large-scale analyses. Using this framework, we analyzed the dynamics of postures and locomotion of wild-type animals and mutants that exhibit severe coiling phenotypes. Our approach can readily be extended to additional automated tracking tasks such as tracking pairs of animals (e.g., for mating assays) or different species.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94. Available: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id.... - PMC - PubMed
-
- White JG, Southgate E, Thomson JN, Brenner S (1986) The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc London Ser B, Biol Sci 314: 1–340. Available: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id.... - PubMed
-
- Bargmann CI, Marder E (2013) From the connectome to brain function. Nat Methods 10: 483–490. Available: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id.... - PubMed
-
- Feng Z, Cronin CJ, Wittig JH, Sternberg PW, Schafer WR (2004) An imaging system for standardized quantitative analysis of C. elegans behavior. BMC Bioinformatics 5: 115 Available: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id.... - PMC - PubMed
-
- Geng W, Cosman P, Berry CC, Feng Z, Schafer WR (2004) Automatic tracking, feature extraction and classification of C elegans phenotypes. IEEE Trans Biomed Eng 51: 1811–1820. Available: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id.... - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

