Bacterial amyloid formation: structural insights into curli biogensis
- PMID: 26439293
- PMCID: PMC4636965
- DOI: 10.1016/j.tim.2015.07.010
Bacterial amyloid formation: structural insights into curli biogensis
Abstract
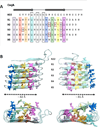
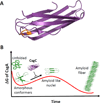
Curli are functional amyloid fibers assembled by many Gram-negative bacteria as part of an extracellular matrix that encapsulates the bacteria within a biofilm. A multicomponent secretion system ensures the safe transport of the aggregation-prone curli subunits across the periplasm and outer membrane, and coordinates subunit self-assembly into surface-attached fibers. To avoid the build-up of potentially toxic intracellular protein aggregates, the timing and location of the interactions of the different curli proteins are of paramount importance. Here we review the structural and molecular biology of curli biogenesis, with a focus on the recent breakthroughs in our understanding of subunit chaperoning and secretion. The mechanistic insight into the curli assembly pathway will provide tools for new biotechnological applications and inform the design of targeted inhibitors of amyloid polymerization and biofilm formation.
Keywords: amyloid chaperone; biofilm matrix; functional amyloid; peptide diffusion channel; protein secretion.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures



References
-
- Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell. Microbiol. 2009;11:1034–1043. - PubMed
-
- Branda SS, et al. Biofilms: the matrix revisited. Trends Microbiol. 2005;13:20–26. - PubMed
-
- Anderson GG, O’Toole GA. Innate and induced resistance mechanisms of bacterial biofilms. Curr. Top. Microbiol. Immunol. 2008;322:85–105. - PubMed
-
- Høiby N, et al. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents. 2010;35:322–332. - PubMed
-
- Gilbert P, et al. The physiology and collective recalcitrance of microbial biofilm communities. Adv. Microb. Physiol. 2002;46:202–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

