Consensus Paper: Cerebellar Development
- PMID: 26439486
- PMCID: PMC4846577
- DOI: 10.1007/s12311-015-0724-2
Consensus Paper: Cerebellar Development
Abstract
The development of the mammalian cerebellum is orchestrated by both cell-autonomous programs and inductive environmental influences. Here, we describe the main processes of cerebellar ontogenesis, highlighting the neurogenic strategies used by developing progenitors, the genetic programs involved in cell fate specification, the progressive changes of structural organization, and some of the better-known abnormalities associated with developmental disorders of the cerebellum.
Keywords: Cerebellum; Differentiation; Progenitors; Purkinje cells; Specification.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



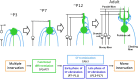
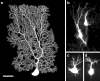



References
-
- Sotelo C, Wassef M. Cerebellar development: afferent organization and Purkinje cell heterogeneity. Philos Trans R Soc Lond B Biol Sci. 1991;331(1261):307–13. - PubMed
-
- Echevarría D, Vieira C, Gimeno L, Martínez S. Neuroepithelial secondary organizers and cell fate specification in the developing brain. Brain Res Brain Res Rev. 2003;43:179–91. - PubMed
-
- Joyner AL, Liu A, Millet S. Otx2, Gbx2 and Fgf8 interact to position and maintain a mid-hindbrain organizer. Curr Opin Cell Biol. 2000;12:736–41. - PubMed
-
- Martinez S, Wassef M, Alvarado-Mallart RM. Induction of a mesencephalic phenotype in the 2-day-old chick prosencephalon is preceded by the early expression of the homeobox gene engrailed. Neuron. 1991;6:971–81. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 NS080390/NS/NINDS NIH HHS/United States
- G0800020/MRC_/Medical Research Council/United Kingdom
- R37 MH085726/MH/NIMH NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 NS092096/NS/NINDS NIH HHS/United States
- R01 NS072441/NS/NINDS NIH HHS/United States
- R01 NS050375/NS/NINDS NIH HHS/United States
- U54 HD083092/HD/NICHD NIH HHS/United States
- R01 NS095733/NS/NINDS NIH HHS/United States
- R01 NS063047/NS/NINDS NIH HHS/United States
- R01 NS089664/NS/NINDS NIH HHS/United States
- R01 NS042205/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

