Comprehensive cancer-gene panels can be used to estimate mutational load and predict clinical benefit to PD-1 blockade in clinical practice
- PMID: 26439694
- PMCID: PMC4741447
- DOI: 10.18632/oncotarget.5950
Comprehensive cancer-gene panels can be used to estimate mutational load and predict clinical benefit to PD-1 blockade in clinical practice
Abstract
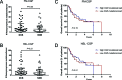
Cancer gene panels (CGPs) are already used in clinical practice to match tumor's genetic profile with available targeted therapies. We aimed to determine if CGPs could also be applied to estimate tumor mutational load and predict clinical benefit to PD-1 and CTLA-4 checkpoint blockade therapy. Whole-exome sequencing (WES) mutation data obtained from melanoma and non-small cell lung cancer (NSCLC) patients published by Snyder et al. 2014 and Rizvi et al. 2015, respectively, were used to select nonsynonymous somatic mutations occurring in genes included in the Foundation Medicine Panel (FM-CGP) and in our own Institutional Panel (HSL-CGP). CGP-mutational load was calculated for each patient using both panels and was associated with clinical outcomes as defined and reported in the original articles. Higher CGP-mutational load was observed in NSCLC patients presenting durable clinical benefit (DCB) to PD-1 blockade (FM-CGP P=0.03, HSL-CGP P=0.01). We also observed that 69% of patients with high CGP-mutational load experienced DCB to PD-1 blockade, as compared to 20% of patients with low CGP-mutational load (FM-CGP and HSL-CGP P=0.01). Noteworthy, predictive accuracy of CGP-mutational load for DCB was not statistically different from that estimated by WES sequencing (P=0.73). Moreover, a high CGP-mutational load was significantly associated with progression-free survival (PFS) in patients treated with PD-1 blockade (FM-CGP P=0.005, HR 0.27, 95% IC 0.105 to 0.669; HSL-CGP P=0.008, HR 0.29, 95% IC 0.116 to 0.719). Similar associations between CGP-mutational load and clinical benefit to CTLA-4 blockade were not observed. In summary, our data reveals that CGPs can be used to estimate mutational load and to predict clinical benefit to PD-1 blockade, with similar accuracy to that reported using WES.
Keywords: PD-1 blockade; cancer-gene panels; immunotherapy; mutational load; response prediction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Shin DS, Ribas A. The evolution of checkpoint blockade as a cancer therapy: what's here, what's next? Curr. Opin. Immunol. 2015;33:23–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

