Low EGFR/MET ratio is associated with resistance to EGFR inhibitors in non-small cell lung cancer
- PMID: 26439803
- PMCID: PMC4741578
- DOI: 10.18632/oncotarget.5131
Low EGFR/MET ratio is associated with resistance to EGFR inhibitors in non-small cell lung cancer
Abstract
Purpose: Although activating mutations in the epidermal growth factor receptor (EGFR) gene are predictive markers for response to EGFR inhibitors, 30-40% of EGFR-mutant non-small cell lung cancer (NSCLC) patients are de novo non-responders. Hence, we sought to explore additional biomarkers of response.
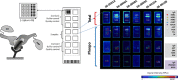
Methods: We conducted a prospective pilot study to characterize the expression and/or activation of key receptor tyrosine kinases (RTKs) in stage IIIB-IV NSCLC tumors. A total of 37 patients were enrolled and 34 underwent EGFR inhibitor treatment.
Results: As expected, patients bearing activating EGFR mutations showed increased progression free survival (PFS) compared to patients with wild-type EGFR status (9.3 vs 1.4 months, p = 0.0629). Analysis of baseline tumor RTK profiles revealed that, regardless of EGFR mutation status, higher levels of EGFR relative to MET correlated with longer PFS. At multiple EGFR/MET ratio cut-offs, including 1, 2 and 3, median PFS according to below vs. above cut-offs were 0.4 vs. 6.1 (p = 0.0001), 0.5 vs. 9.3 (p = 0.0006) and 1.0 vs. 11.2 months (p = 0.0008), respectively.
Conclusion: The EGFR/MET ratio measured in tumors at baseline may help identify NSCLC patients most likely to benefit from prolonged PFS when treated with EGFR inhibitors.
Keywords: EGFR TKI; EGFR/MET ratio; HER3; NSCLC; PFS.
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures


References
-
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. - PubMed
-
- Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. - PubMed
-
- Gridelli C, De Marinis F, Di Maio M, Cortinovis D, Cappuzzo F, Mok T. Gefitinib as first-line treatment for patients with advanced non-small-cell lung cancer with activating epidermal growth factor receptor mutation: Review of the evidence. Lung Cancer. 2011;71:249–257. - PubMed
-
- Besse B, Adjei A, Baas P, Meldgaard P, Nicolson M, Paz-Ares L, Reck M, Smit EF, Syrigos K, Stahel R, Felip E, Peters S, Panel M. 2nd ESMO Consensus Conference on Lung Cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann Oncol. 2014;25:1475–1484. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

