AMPK Protects Leukemia-Initiating Cells in Myeloid Leukemias from Metabolic Stress in the Bone Marrow
- PMID: 26440282
- PMCID: PMC4597792
- DOI: 10.1016/j.stem.2015.08.019
AMPK Protects Leukemia-Initiating Cells in Myeloid Leukemias from Metabolic Stress in the Bone Marrow
Abstract
How cancer cells adapt to metabolically adverse conditions in patients and strive to proliferate is a fundamental question in cancer biology. Here we show that AMP-activated protein kinase (AMPK), a metabolic checkpoint kinase, confers metabolic stress resistance to leukemia-initiating cells (LICs) and promotes leukemogenesis. Upon dietary restriction, MLL-AF9-induced murine acute myeloid leukemia (AML) activated AMPK and maintained leukemogenic potential. AMPK deletion significantly delayed leukemogenesis and depleted LICs by reducing the expression of glucose transporter 1 (Glut1), compromising glucose flux, and increasing oxidative stress and DNA damage. LICs were particularly dependent on AMPK to suppress oxidative stress in the hypoglycemic bone marrow environment. Strikingly, AMPK inhibition synergized with physiological metabolic stress caused by dietary restriction and profoundly suppressed leukemogenesis. Our results indicate that AMPK protects LICs from metabolic stress and that combining AMPK inhibition with physiological metabolic stress potently suppresses AML by inducing oxidative stress and DNA damage.
Keywords: AML; AMPK; DNA damage; ROS; dietary restriction; glycolysis; leukemia-initiating cells; metabolic stress.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
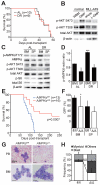
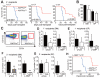

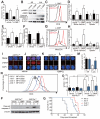
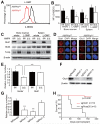
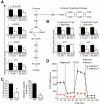
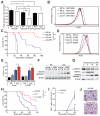
Comment in
-
AMPK Keeps Tumor Cells from Starving to Death.Cell Stem Cell. 2015 Nov 5;17(5):503-4. doi: 10.1016/j.stem.2015.10.007. Cell Stem Cell. 2015. PMID: 26544110
References
-
- Almeida A, Moncada S, Bolanos JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol. 2004;6:45–51. - PubMed
-
- Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LG, Foufelle F, Carling D, Hardie DG, Baldwin SA. Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK) J Cell Sci. 2002;115:2433–2442. - PubMed
-
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

