Targeted massively parallel sequencing of angiosarcomas reveals frequent activation of the mitogen activated protein kinase pathway
- PMID: 26440310
- PMCID: PMC4742160
- DOI: 10.18632/oncotarget.5936
Targeted massively parallel sequencing of angiosarcomas reveals frequent activation of the mitogen activated protein kinase pathway
Abstract
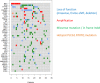
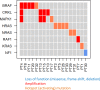
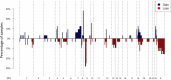
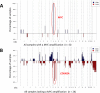
Angiosarcomas are rare malignant mesenchymal tumors of endothelial differentiation. The clinical behavior is usually aggressive and the prognosis for patients with advanced disease is poor with no effective therapies. The genetic bases of these tumors have been partially revealed in recent studies reporting genetic alterations such as amplifications of MYC (primarily in radiation-associated angiosarcomas), inactivating mutations in PTPRB and R707Q hotspot mutations of PLCG1. Here, we performed a comprehensive genomic analysis of 34 angiosarcomas using a clinically-approved, hybridization-based targeted next-generation sequencing assay for 341 well-established oncogenes and tumor suppressor genes. Over half of the angiosarcomas (n = 18, 53%) harbored genetic alterations affecting the MAPK pathway, involving mutations in KRAS, HRAS, NRAS, BRAF, MAPK1 and NF1, or amplifications in MAPK1/CRKL, CRAF or BRAF. The most frequently detected genetic aberrations were mutations in TP53 in 12 tumors(35%) and losses of CDKN2A in9 tumors (26%). MYC amplifications were generally mutually exclusive of TP53 alterations and CDKN2A loss and were identified in 8 tumors (24%), most of which (n = 7, 88%) arose post-irradiation. Previously reported mutations in PTPRB (n = 10, 29%) and one (3%) PLCG1 R707Q mutation were also identified. Our results demonstrate that angiosarcomas are a genetically heterogeneous group of tumors, harboring a wide range of genetic alterations. The high frequency of genetic events affecting the MAPK pathway suggests that targeted therapies inhibiting MAPK signaling may be promising therapeutic avenues in patients with advanced angiosarcomas.
Keywords: MAPK pathway; MYC; angiosarcoma; genetics.
Conflict of interest statement
Bastian Schilling has received honoraria from Roche and travel support from Bristol-Meyers Squibb. Dirk Schadendorf is on the advisory board or has received honoraria from Roche, Genentech, Novartis, Amgen, GlaxoSmithKline, Bristol-Myers Squibb, Boehringer Ingelheim, and Merck. The remaining authors have no disclosures
Figures

References
-
- Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ. Angiosarcoma. The lancet oncology. 2010;11:983–991. - PubMed
-
- Frith AE, Hirbe AC, Van Tine BA. Novel pathways and molecular targets for the treatment of sarcoma. Current oncology reports. 2013;15:378–385. - PubMed
-
- Maki RG, D'Adamo DR, Keohan ML, Saulle M, Schuetze SM, Undevia SD, Livingston MB, Cooney MM, Hensley ML, Mita MM, Takimoto CH, Kraft AS, Elias AD, Brockstein B, Blachere NE, Edgar MA, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:3133–3140. - PMC - PubMed
-
- Ray-Coquard I, Italiano A, Bompas E, Le Cesne A, Robin YM, Chevreau C, Bay JO, Bousquet G, Piperno-Neumann S, Isambert N, Lemaitre L, Fournier C, Gauthier E, Collard O, Cupissol D, Clisant S, et al. Sorafenib for patients with advanced angiosarcoma: a phase II Trial from the French Sarcoma Group (GSF/GETO) The oncologist. 2012;17:260–266. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

