Multispectral tissue characterization for intestinal anastomosis optimization
- PMID: 26440616
- PMCID: PMC5996867
- DOI: 10.1117/1.JBO.20.10.106001
Multispectral tissue characterization for intestinal anastomosis optimization
Abstract
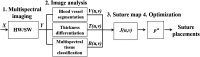
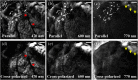
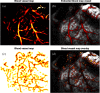
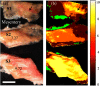
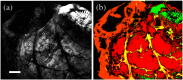
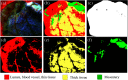
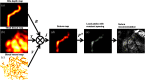
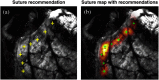
Intestinal anastomosis is a surgical procedure that restores bowel continuity after surgical resection to treat intestinal malignancy, inflammation, or obstruction. Despite the routine nature of intestinal anastomosis procedures, the rate of complications is high. Standard visual inspection cannot distinguish the tissue subsurface and small changes in spectral characteristics of the tissue, so existing tissue anastomosis techniques that rely on human vision to guide suturing could lead to problems such as bleeding and leakage from suturing sites. We present a proof-of-concept study using a portable multispectral imaging (MSI) platform for tissue characterization and preoperative surgical planning in intestinal anastomosis. The platform is composed of a fiber ring light-guided MSI system coupled with polarizers and image analysis software. The system is tested on ex vivo porcine intestine tissue, and we demonstrate the feasibility of identifying optimal regions for suture placement.
Figures