μOrgano: A Lego®-Like Plug & Play System for Modular Multi-Organ-Chips
- PMID: 26440672
- PMCID: PMC4595286
- DOI: 10.1371/journal.pone.0139587
μOrgano: A Lego®-Like Plug & Play System for Modular Multi-Organ-Chips
Abstract
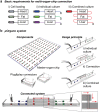
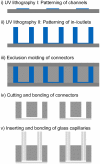
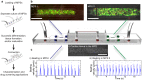
Human organ-on-a-chip systems for drug screening have evolved as feasible alternatives to animal models, which are unreliable, expensive, and at times erroneous. While chips featuring single organs can be of great use for both pharmaceutical testing and basic organ-level studies, the huge potential of the organ-on-a-chip technology is revealed by connecting multiple organs on one chip to create a single integrated system for sophisticated fundamental biological studies and devising therapies for disease. Furthermore, since most organ-on-a-chip systems require special protocols with organ-specific media for the differentiation and maturation of the tissues, multi-organ systems will need to be temporally customizable and flexible in terms of the time point of connection of the individual organ units. We present a customizable Lego®-like plug & play system, μOrgano, which enables initial individual culture of single organ-on-a-chip systems and subsequent connection to create integrated multi-organ microphysiological systems. As a proof of concept, the μOrgano system was used to connect multiple heart chips in series with excellent cell viability and spontaneously physiological beat rates.
Conflict of interest statement
Figures

References
-
- Marx U, Sandig V. Drug Testing In Vitro: Breakthroughs and Trends in Cell Culture Technology. 1st ed Weinheim: Wiley-VCH; 2007.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

