Iridium-Catalyzed Enantioselective Allylic Substitution of Enol Silanes from Vinylogous Esters and Amides
- PMID: 26441002
- PMCID: PMC5342901
- DOI: 10.1021/jacs.5b09980
Iridium-Catalyzed Enantioselective Allylic Substitution of Enol Silanes from Vinylogous Esters and Amides
Abstract
The enol silanes of vinylogous esters and amides are classic dienes for Diels-Alder reactions. Here, we report their reactivity as nucleophiles in Ir-catalyzed, enantioselective allylic substitution reactions. A variety of allylic carbonates react with these nucleophiles to give allylated products in good yields with high enantioselectivities and excellent branched-to-linear ratios. These reactions occur with KF or alkoxide as the additive, but mechanistic studies suggest that these additives do not activate the enol silanes. Instead, they serve as bases to promote the cyclometalation to generate the active Ir catalyst. The carbonate anion, which was generated from the oxidative addition of the allylic carbonate, likely activates the enol silanes to trigger their activity as nucleophiles for reactions with the allyliridium electrophile. The synthetic utility of this method was illustrated by the synthesis of the anti-muscarinic drug, fesoterodine.
Figures


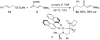


References
-
- Danishefsky SJ, Kitahara T. J Am Chem Soc. 1974;96:7807.
-
- Kozmin SA, Rawal VH. J Org Chem. 1997;62:5252.
-
- Kozmin SA, Green MT, Rawal VH. J Org Chem. 1999;64:8045. - PubMed
- Huang Y, Rawal VH. J Am Chem Soc. 2002;124:9662. - PubMed
- Huang Y, Unni AK, Thadani AN, Rawal VH. Nature. 2003;424:146. - PubMed
- Unni AK, Takenaka N, Yamamoto H, Rawal VH. J Am Chem Soc. 2005;127:1336. - PubMed
- Panarese JD, Waters SP. Org Lett. 2009;11:5086. - PMC - PubMed
- Zhang Y, Danishefsky SJ. J Am Chem Soc. 2010;132:9567. - PMC - PubMed
-
- Acocella MR, De Rosa M, Massa A, Palombi L, Villano R, Scettri A. Tetrahedron. 2005;61:4091.
- Attanasi OA, Favi G, Filippone P, Giorgi G, Mantellini F, Moscatelli G, Spinelli D. Org Lett. 2008;10:1983. - PubMed
- De Rosa M, Soriente A. Tetrahedron. 2011;67:5949.
-
- Liu Y, Bakshi K, Zavalij P, Doyle MP. Org Lett. 2010;12:4304. - PMC - PubMed
- Ohtani T, Tsukamoto S, Kanda H, Misawa K, Urakawa Y, Fujimaki T, Imoto M, Takahashi Y, Takahashi D, Toshima K. Org Lett. 2010;12:5068. - PubMed
- Guéret SM, Furkert DP, Brimble MA. Org Lett. 2010;12:5226. - PubMed
- Hiraoka S, Harada S, Nishida A. J Org Chem. 2010;75:3871. - PubMed
- Fujiwara K, Tanaka K, Katagiri Y, Kawai H, Suzuki T. Tetrahedron Lett. 2010;51:4543.
- Liu Y, Doyle MP. Org Biomol Chem. 2012;10:6388. - PubMed
- Kondratov IS, Dolovanyuk VG, Tolmachova NA, Gerus II, Bergander K, Fröhlich R, Haufe G. Org Biomol Chem. 2012;10:8778. - PubMed
- Li B, Widlicka D, Boucher S, Hayward C, Lucas J, Murray JC, O'Neil BT, Pfisterer D, Samp L, VanAlsten J, Xiang YQ, Young J. Org Process Res Dev. 2012;16:2031.
- Edwankar RV, Edwankar CR, Namjoshi OA, Deschamps JR, Cook JM. J Nat Prod. 2012;75:181. - PMC - PubMed
- Arnold DM, LaPorte MG, Anderson SM, Wipf P. Tetrahedron. 2013;69:7719. - PMC - PubMed
- Desrosiers JN, Kelly CB, Fandrick DR, Nummy L, Campbell SJ, Wei X, Sarvestani M, Lee H, Sienkiewicz A, Sanyal S, Zeng X, Grinberg N, Ma S, Song JJ, Senanayake CH. Org Lett. 2014;16:1724. - PubMed
- Wu B, He S, Wu Xd, Pan Yj. Planta Med. 2006;72:1334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

