Structure of RagB, a major immunodominant outer-membrane surface receptor antigen of Porphyromonas gingivalis
- PMID: 26441291
- PMCID: PMC4823178
- DOI: 10.1111/omi.12140
Structure of RagB, a major immunodominant outer-membrane surface receptor antigen of Porphyromonas gingivalis
Abstract
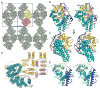
Porphyromonas gingivalis is the main causative agent of periodontitis. It deregulates the inflammatory and innate host immune responses through virulence factors, which include the immunodominant outer-membrane surface receptor antigens A (PgRagA) and B (PgRagB), co-transcribed from the rag pathogenicity island. The former is predicted to be a Ton-dependent porin-type translocator but the targets of this translocation and the molecular function of PgRagB are unknown. Phenomenologically, PgRagB has been linked with epithelial cell invasion and virulence according to murine models. It also acts as a Toll-like receptor agonist and promotes multiple mediators of inflammation. Hence, PgRagB is a candidate for the development of a periodontitis vaccine, which would be facilitated by the knowledge of its atomic structure. Here, we crystallized and solved the structure of 54-kDa PgRagB, which revealed a single domain centered on a curved helical scaffold. It consists of four tetratrico peptide repeats (TPR1-4), each arranged as two helices connected by a linker, plus two extra downstream capping helices. The concave surface bears four large intertwined irregular inserts (A-D), which contribute to an overall compact moiety. Overall, PgRagB shows substantial structural similarity with Bacteroides thetaiotaomicron SusD and Tannerella forsythia NanU, which are, respectively, engaged in binding and uptake of malto-oligosaccharide/starch and sialic acid. This suggests a similar sugar-binding function for PgRagB for uptake by the cognate PgRagA translocator, and, consistently, three potential monosaccharide-binding sites were tentatively assigned on the molecular surface.
Keywords: SusD-like proteins; X-ray crystal structure; periodontitis; sugar-binding proteins; tetratricorepeat proteins.
© 2015 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

