ITPA Polymorphisms Are Associated with Hematological Side Effects during Antiviral Therapy for Chronic HCV Infection
- PMID: 26441325
- PMCID: PMC4595504
- DOI: 10.1371/journal.pone.0139317
ITPA Polymorphisms Are Associated with Hematological Side Effects during Antiviral Therapy for Chronic HCV Infection
Abstract
Background/objective: Genetic polymorphisms in the inosine triphosphatase (ITPA) gene have been associated with the protection from early ribavirin(RBV)-induced hemolytic anemia among patients with chronic hepatitis C virus (HCV) infection. The aim of the present study was to investigate the association between the functional ITPA variants and hematological side effects during antiviral therapy with pegylated interferon (PegIFN) and RBV.
Patients and methods: This cohort study included all consecutive Caucasian patients treated for chronic HCV infection with PegIFN and RBV between 2000 and 2009 for whom a serum sample was available for genetic testing. The predicted inosine triphosphate pyrophosphatase (ITPase) activity was based on the genotypes of the SNPs rs1127354 and rs7270101. Decline in hemoglobin (Hb) during antiviral therapy, as well as dose reductions, blood transfusions and use of erythropoietin were assessed.
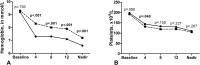
Results: In total, 213 patients were included. The predicted ITPase activity was normal among 152 (71%) patients; 61 (29%) patients had ITPase deficiency. By multivariable linear regression, RBV dose in mg per kilogram (Beta 0.09, 95%CI 0.04-0.13, p<0.001) and normal ITPase activity (Beta 0.89, 95%CI 0.64-1.14, p<0.001) were associated with more Hb decline at week 4 of treatment. Patients with normal ITPase activity underwent more dose adjustments of RBV than patients with ITPase deficiency (19(13%) vs 1(2%),p = 0.014) and received erythropoietin more frequently (12 (8%) vs 0 (0%),p = 0.024).
Conclusion: Genetic variants in the ITPA gene protected against RBV treatment-induced anemia among Caucasian patients with chronic HCV infection. Patients with normal ITPase activity underwent more dose reductions of RBV and received erythropoietin more frequently.
Conflict of interest statement
Figures

References
-
- Russo MW, Fried MW. Side effects of therapy for chronic hepatitis C. Gastroenterology. 2003;124(6):1711–9. Epub 2003/05/23. S0016508503003949 [pii]. . - PubMed
-
- McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123(4):1061–9. Epub 2002/10/03. S0016508502002111 [pii]. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

