Cell-to-cell infection by HIV contributes over half of virus infection
- PMID: 26441404
- PMCID: PMC4592948
- DOI: 10.7554/eLife.08150
Cell-to-cell infection by HIV contributes over half of virus infection
Abstract
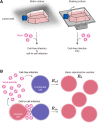
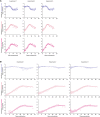
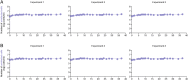
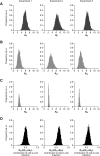
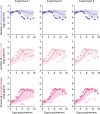
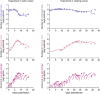
Cell-to-cell viral infection, in which viruses spread through contact of infected cell with surrounding uninfected cells, has been considered as a critical mode of virus infection. However, since it is technically difficult to experimentally discriminate the two modes of viral infection, namely cell-free infection and cell-to-cell infection, the quantitative information that underlies cell-to-cell infection has yet to be elucidated, and its impact on virus spread remains unclear. To address this fundamental question in virology, we quantitatively analyzed the dynamics of cell-to-cell and cell-free human immunodeficiency virus type 1 (HIV-1) infections through experimental-mathematical investigation. Our analyses demonstrated that the cell-to-cell infection mode accounts for approximately 60% of viral infection, and this infection mode shortens the generation time of viruses by 0.9 times and increases the viral fitness by 3.9 times. Our results suggest that even a complete block of the cell-free infection would provide only a limited impact on HIV-1 spread.
Keywords: HIV-1; basic reproduction number; cell-free infection; cell-to-cell infection; computational biology; infectious disease; mathematical model; microbiology; systems biology; viruses.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures