Histopathology and oxidative stress analysis of concomitant misoprostol and celecoxib administration
- PMID: 26441478
- PMCID: PMC4588210
- DOI: 10.1293/tox.2015-0016
Histopathology and oxidative stress analysis of concomitant misoprostol and celecoxib administration
Erratum in
-
Errata (Printer's correction).J Toxicol Pathol. 2016 Jan;29(1):74. Epub 2016 Feb 17. J Toxicol Pathol. 2016. PMID: 26989306 Free PMC article.
Abstract
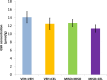
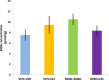
Nonsteroidal anti-inflammatory drugs (NSAIDs), non-selective or selective inhibitors of cyclooxygenase (COX-1 and -2), reduce pain and inflammation associated with arthritic diseases. Celecoxib, a COX-2-selective inhibitor providing decreased gastric injury relative to non-selective NSAIDs, is commonly prescribed. Misoprostol, a prostaglandin analog, supplements NSAID-inhibited prostaglandin levels. As concomitant celecoxib and misoprostol administration has been shown to intensify renal adverse effects, this article examined the influence of concomitant administration on hepatic histopathology, oxidative stress, and celecoxib concentration. On days 1 and 2, rat groups (n = 6) were gavaged twice daily (two groups with vehicle and two groups with 100 μg/kg misoprostol). From day 3 to day 9, one celecoxib dose (40 mg/kg) replaced a vehicle dose of one group and one group received celecoxib in addition to misoprostol. Livers were harvested on day 10. No hepatic abnormalities were observed denoting a lack of influence by either drug. Also no change in mean biomarker levels was detected. The changes in hepatic celecoxib concentration in the misoprostol-receiving group compared to control were not significant. Thus misoprostol does not influence hepatic celecoxib effects in terms of histopathology, oxidative stress, or celecoxib concentration level at the dosage and duration examined.
Keywords: NSAIDs; celecoxib; glutathione; histopathology; malondialdehyde; misoprostol.
Figures

References
-
- Chakraborty S, Kar SK, Roy K, and Sengupta C. Exploring effects of different nonsteroidal antiinflammatory drugs on malondialdehyde profile. Acta Pol Pharm. 63: 83–88. 2006. - PubMed
-
- Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, Burr AM, Zhao WW, Kent JD, Lefkowith JB, Verburg KM, and Geis GS. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 284: 1247–1255. 2000. - PubMed
-
- Cooper DL, Wood RC, 3rd , Wyatt JE, and Harirforoosh S. Pharmacokinetic interactions between rebamipide and selected nonsteroidal anti-inflammatory drugs in rats. Eur J Pharm Sci. 53: 28–34. 2014. - PubMed
-
- Buttgereit F, Burmester GR, and Simon LS. Gastrointestinal toxic side effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2-specific inhibitors. Am J Med. 110(Suppl 3A): 13S–19S. 2001. - PubMed
-
- Paulson SK, Zhang JY, Breau AP, Hribar JD, Liu NW, Jessen SM, Lawal YM, Cogburn JN, Gresk CJ, Markos CS, Maziasz TJ, Schoenhard GL, and Burton EG. Pharmacokinetics, tissue distribution, metabolism, and excretion of celecoxib in rats. Drug Metab Dispos. 28: 514–521. 2000. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
