An array of highly flexible electrodes with a tailored configuration locked by gelatin during implantation-initial evaluation in cortex cerebri of awake rats
- PMID: 26441505
- PMCID: PMC4585103
- DOI: 10.3389/fnins.2015.00331
An array of highly flexible electrodes with a tailored configuration locked by gelatin during implantation-initial evaluation in cortex cerebri of awake rats
Abstract
Background: A major challenge in the field of neural interfaces is to overcome the problem of poor stability of neuronal recordings, which impedes long-term studies of individual neurons in the brain. Conceivably, unstable recordings reflect relative movements between electrode and tissue. To address this challenge, we have developed a new ultra-flexible electrode array and evaluated its performance in awake non-restrained animals.
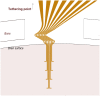
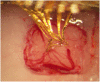
Methods: An array of eight separated gold leads (4 × 10 μm), individually flexible in 3D, were cut from a gold sheet using laser milling and insulated with Parylene C. To provide structural support during implantation into rat cortex, the electrode array was embedded in a hard gelatin based material, which dissolves after implantation. Recordings were made during 3 weeks. At termination, the animals were perfused with fixative and frozen to prevent dislocation of the implanted electrodes. A thick slice of brain tissue, with the electrode array still in situ, was made transparent using methyl salicylate to evaluate the conformation of the implanted electrode array.
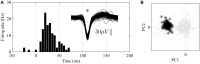
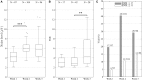
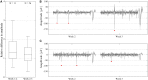
Results: Median noise levels and signal/noise remained relatively stable during the 3 week observation period; 4.3-5.9 μV and 2.8-4.2, respectively. The spike amplitudes were often quite stable within recording sessions and for 15% of recordings where single-units were identified, the highest-SNR unit had an amplitude higher than 150 μV. In addition, high correlations (>0.96) between unit waveforms recorded at different time points were obtained for 58% of the electrode sites. The structure of the electrode array was well preserved 3 weeks after implantation.
Conclusions: A new implantable multichannel neural interface, comprising electrodes individually flexible in 3D that retain its architecture and functionality after implantation has been developed. Since the new neural interface design is adaptable, it offers a versatile tool to explore the function of various brain structures.
Keywords: brain machine interface; brain micro-motions; embedding material; flexible electronic implant; mechanical compliance; neural probe; neuromodulation; stable neural recordings.
Figures


References
-
- Andrei A., Tutunjyan N., Verbinnen G., Vanput S., Krylychkina O., Eberle W., et al. (2012). Fabrication and successful in-vivo implantation of a flexible neural implant with a hybrid polyimide-silicon design, in Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS (San Diego, CA: ), 3890–3893. - PubMed
-
- Bae W. J., Ruddy B. P., Richardson A. G., Hunter I. W., Bizzi E. (2008). Cortical recording with polypyrrole microwire electrodes, in Conference Proceedings?: Annual International Conference of the IEEE Engineering in Medicine and Biology Society (Vancouver, BC: ), 5794–5797. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

