Targeting protein-protein interactions in complexes organized by A kinase anchoring proteins
- PMID: 26441649
- PMCID: PMC4562273
- DOI: 10.3389/fphar.2015.00192
Targeting protein-protein interactions in complexes organized by A kinase anchoring proteins
Abstract
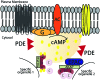
Cyclic AMP is a ubiquitous intracellular second messenger involved in the regulation of a wide variety of cellular processes, a majority of which act through the cAMP - protein kinase A (PKA) signaling pathway and involve PKA phosphorylation of specific substrates. PKA phosphorylation events are typically spatially restricted and temporally well controlled. A-kinase anchoring proteins (AKAPs) directly bind PKA and recruit it to specific subcellular loci targeting the kinase activity toward particular substrates, and thereby provide discrete spatiotemporal control of downstream phosphorylation events. AKAPs also scaffold other signaling molecules into multi-protein complexes that function as crossroads between different signaling pathways. Targeting AKAP coordinated protein complexes with high-affinity peptidomimetics or small molecules to tease apart distinct protein-protein interactions (PPIs) therefore offers important means to disrupt binding of specific components of the complex to better understand the molecular mechanisms involved in the function of individual signalosomes and their pathophysiological role. Furthermore, development of novel classes of small molecules involved in displacement of AKAP-bound signal molecules is now emerging. Here, we will focus on mechanisms for targeting PPI, disruptors that modulate downstream cAMP signaling and their role, especially in the heart.
Keywords: AKAP; cAMP; disruptor peptide; heart; protein–protein interaction; small molecule.
Figures


References
-
- Ahmad F., Shen W., Vandeput F., Szabo-Fresnais N., Krall J., Degerman E., et al. (2015). Regulation of sarcoplasmic reticulum Ca2+ ATPase 2 (SERCA2) activity by phosphodiesterase 3A (PDE3A) in human myocardium: phosphorylation-dependent interaction of PDE3A1 with SERCA2. J. Biol. Chem. 290 6763–6776. 10.1074/jbc.M115.638585 - DOI - PMC - PubMed
-
- Alto N. M., Soderling S. H., Hoshi N., Langeberg L. K., Fayos R., Jennings P. A., et al. (2003). Bioinformatic design of A-kinase anchoring protein-in silico: a potent and selective peptide antagonist of type II protein kinase A anchoring. Proc. Natl. Acad. Sci. U.S.A. 100 4445–4450. 10.1073/pnas.0330734100 - DOI - PMC - PubMed
-
- An S. S., Askovich P. S., Zarembinski T. I., Ahn K., Peltier J. M., von Rechenberg M., et al. (2011). A novel small molecule target in human airway smooth muscle for potential treatment of obstructive lung diseases: a staged high-throughput biophysical screening. Respir. Res. 12 8 10.1186/1465-9921-12-8 - DOI - PMC - PubMed
-
- Appert-Collin A., Cotecchia S., Nenniger-Tosato M., Pedrazzini T., Diviani D. (2007). The A-kinase anchoring protein (AKAP)-Lbc-signaling complex mediates alpha1 adrenergic receptor-induced cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. U.S.A. 104 10140–10145. 10.1073/pnas.0701099104 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

