Genetically-encoded tools for cAMP probing and modulation in living systems
- PMID: 26441653
- PMCID: PMC4569861
- DOI: 10.3389/fphar.2015.00196
Genetically-encoded tools for cAMP probing and modulation in living systems
Abstract
Intracellular 3'-5'-cyclic adenosine monophosphate (cAMP) is one of the principal second messengers downstream of a manifold of signal transduction pathways, including the ones triggered by G protein-coupled receptors. Not surprisingly, biochemical assays for cAMP have been instrumental for basic research and drug discovery for decades, providing insights into cellular physiology and guiding pharmaceutical industry. However, despite impressive track record, the majority of conventional biochemical tools for cAMP probing share the same fundamental shortcoming-all the measurements require sample disruption for cAMP liberation. This common bottleneck, together with inherently low spatial resolution of measurements (as cAMP is typically analyzed in lysates of thousands of cells), underpin the ensuing limitations of the conventional cAMP assays: (1) genuine kinetic measurements of cAMP levels over time in a single given sample are unfeasible; (2) inability to obtain precise information on cAMP spatial distribution and transfer at subcellular levels, let alone the attempts to pinpoint dynamic interactions of cAMP and its effectors. At the same time, tremendous progress in synthetic biology over the recent years culminated in drastic refinement of our toolbox, allowing us not only to bypass the limitations of conventional assays, but to put intracellular cAMP life-span under tight control-something, that seemed scarcely attainable before. In this review article we discuss the main classes of modern genetically-encoded tools tailored for cAMP probing and modulation in living systems. We examine the capabilities and weaknesses of these different tools in the context of their operational characteristics and applicability to various experimental set-ups involving living cells, providing the guidance for rational selection of the best tools for particular needs.
Keywords: biosensor; cAMP signaling; cell-based assays; cyclic nucleotide; genetically encoded probe; optogenetics.
Figures
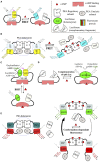
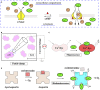
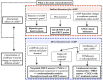
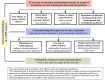
References
-
- Akimoto M., Zhang Z., Boulton S., Selvaratnam R., Van Schouwen B., Gloyd M., et al. (2014). A mechanism for the auto-inhibition of hyperpolarization-activated cyclic nucleotide-gated (HCN) channel opening and its relief by cAMP. J. Biol. Chem. 289, 22205–22220. 10.1074/jbc.M114.572164 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

