HIV-1 Tat and cocaine mediated synaptopathy in cortical and midbrain neurons is prevented by the isoflavone Equol
- PMID: 26441850
- PMCID: PMC4561964
- DOI: 10.3389/fmicb.2015.00894
HIV-1 Tat and cocaine mediated synaptopathy in cortical and midbrain neurons is prevented by the isoflavone Equol
Abstract
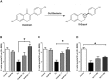
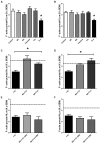
Illicit drugs, such as cocaine, are known to increase the likelihood and severity of HIV-1 associated neurocognitive disorders (HAND). In the current studies synaptic integrity was assessed following exposure to low concentrations of the HIV-1 viral protein Tat 1-86B, with or without cocaine, by quantifying filamentous actin (F-actin) rich structures (i.e., puncta and dendritic spines) on neuronal dendrites in vitro. In addition, the synapse-protective effects of either R-Equol (RE) or S-Equol (SE; derivatives of the soy isoflavone, daidzein) were determined. Individually, neither low concentrations of HIV-1 Tat (10 nM) nor low concentrations of cocaine (1.6 μM) had any significant effect on F-actin puncta number; however, the same low concentrations of HIV-1 Tat + cocaine in combination significantly reduced dendritic synapses. This synaptic reduction was prevented by pre-treatment with either RE or SE, in an estrogen receptor beta dependent manner. In sum, targeted therapeutic intervention with SE may prevent HIV-1 + drug abuse synaptopathy, and thereby potentially influence the development of HAND.
Keywords: F-actin; HAND; S-Equol; estrogen receptor; soy isoflavone.
Figures

References
-
- Adams S. M., Aksenova M. V., Aksenov M. Y., Mactutus C. F., Booze R. M. (2012). Soy isoflavones genistein and daidzein exert anti-apoptotic actions via a selective er-mediated mechanism in neurons following hiv-1 tat(1-86) exposure. PLoS ONE 7:e37540 10.1371/journal.pone.0037540 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

