Interactive effects of cocaine on HIV infection: implication in HIV-associated neurocognitive disorder and neuroAIDS
- PMID: 26441868
- PMCID: PMC4562305
- DOI: 10.3389/fmicb.2015.00931
Interactive effects of cocaine on HIV infection: implication in HIV-associated neurocognitive disorder and neuroAIDS
Abstract
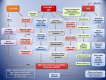
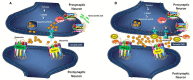
Substantial epidemiological studies suggest that not only, being one of the reasons for the transmission of the human immunodeficiency virus (HIV), but drug abuse also serves its role in determining the disease progression and severity among the HIV infected population. This article focuses on the drug cocaine, and its role in facilitating entry of HIV into the CNS and mechanisms of development of neurologic complications in infected individuals. Cocaine is a powerfully addictive central nervous system stimulating drug, which increases the level of neurotransmitter dopamine (DA) in the brain, by blocking the dopamine transporters (DAT) which is critical for DA homeostasis and neurocognitive function. Tat protein of HIV acts as an allosteric modulator of DAT, where as cocaine acts as reuptake inhibitor. When macrophages in the CNS are exposed to DA, their number increases. These macrophages release inflammatory mediators and neurotoxins, causing chronic neuroinflammation. Cocaine abuse during HIV infection enhances the production of platelet monocyte complexes (PMCs), which may cross transendothelial barrier, and result in HIV-associated neurocognitive disorder (HAND). HAND is characterized by neuroinflammation, including astrogliosis, multinucleated giant cells, and neuronal apoptosis that is linked to progressive virus infection and immune deterioration. Cocaine and viral proteins are capable of eliciting signaling transduction pathways in neurons, involving in mitochondrial membrane potential loss, oxidative stress, activation of JNK, p38, and ERK/MAPK pathways, and results in downstream activation of NF-κB that leads to HAND. Tat-induced inflammation provokes permeability of the blood brain barrier (BBB) in the platelet dependent manner, which can potentially be the reason for progression to HAND during HIV infection. A better understanding on the role of cocaine in HIV infection can give a clue in developing novel therapeutic strategies against HIV-1 infection in cocaine using HIV infected population.
Keywords: AIDS; CNS; HAND; HIV; blood brain barrier; cocaine; neuroAIDS.
Figures
References
-
- Addai A. B., Pandhare J., Mantri C. K., Dash C. (2014). Cocaine enhances HIV-1 integration in CD4+ T cells by modulating the epigenetic DNA signatures of host genome. FASEB. J. 28.776.3.. - PubMed
-
- Anthony J. C., Vlahov D., Nelson K. E., Cohn S., Astemborski J., Solomon L. (1991). New evidence on intravenous cocaine use and the risk of infection with human immunodeficiency virus type 1. Am. J. Epidemiol. 134, 1175–1189. - PubMed
-
- Atluri V. S., Pilakka-Kanthikeel S., Samikkannu T., Sagar V., Kurapati K. R., Saxena S. K. (2014). Vorinostat positively regulates synaptic plasticity genes expression and spine density in HIV infected neurons: role of nicotine in progression of HIV-associated neurocognitive disorder. Mol. Brain 7:37. 10.1186/1756-6606-7-37 - DOI - PMC - PubMed
-
- Chaisson R. E., Bacchetti P., Osmond D., Brodie B., Sande M. A., Moss A. R. (1989). Cocaine use and HIV infection in intravenous drug users in San Francisco. JAMA 261, 561–565. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

