Biogeography of photoautotrophs in the high polar biome
- PMID: 26442009
- PMCID: PMC4566839
- DOI: 10.3389/fpls.2015.00692
Biogeography of photoautotrophs in the high polar biome
Abstract
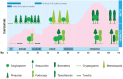
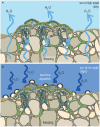
The global latitudinal gradient in biodiversity weakens in the high polar biome and so an alternative explanation for distribution of Arctic and Antarctic photoautotrophs is required. Here we identify how temporal, microclimate and evolutionary drivers of biogeography are important, rather than the macroclimate features that drive plant diversity patterns elsewhere. High polar ecosystems are biologically unique, with a more central role for bryophytes, lichens and microbial photoautotrophs over that of vascular plants. Constraints on vascular plants arise mainly due to stature and ontogenetic barriers. Conversely non-vascular plant and microbial photoautotroph distribution is correlated with favorable microclimates and the capacity for poikilohydric dormancy. Contemporary distribution also depends on evolutionary history, with adaptive and dispersal traits as well as legacy influencing biogeography. We highlight the relevance of these findings to predicting future impacts on diversity of polar photoautotrophs and to the current status of plants in Arctic and Antarctic conservation policy frameworks.
Keywords: antarctic; arctic; bryophytes; cryptogams; cyanobacteria; lichen; plant biogeography.
Figures



References
-
- Aleksandrova V. D. (1988). Vegetation of the Soviet Polar Deserts. Cambridge: Cambridge University Press.
-
- Ballantyne A., Greenwood D., Sinninghe Damste J., Csank A., Eberle J., Rybczynski N. (2010). Arctic surface temperatures during the Pliocene indicated by multiple independent proxies. Geology 38, 603–606. 10.1130/G30815.1 - DOI
-
- Bay C. (1992). A phytogeographical study of the vascular plants of northern Greenland. Bioscience 36, 1–120.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

