Metabolite profiling and transcript analysis reveal specificities in the response of a berry derived cell culture to abiotic stresses
- PMID: 26442042
- PMCID: PMC4585150
- DOI: 10.3389/fpls.2015.00728
Metabolite profiling and transcript analysis reveal specificities in the response of a berry derived cell culture to abiotic stresses
Abstract
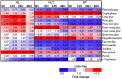
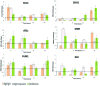
As climate changes, there is a need to understand the expected effects on viticulture. In nature, stresses exist in a combined manner, hampering the elucidation of the effect of individual cues on grape berry metabolism. Cell suspension culture originated from pea-size Gamy Red grape berry was used to harness metabolic response to high light (HL; 2500 μmol m(-2)s(-1)), high temperature (HT; 40°C) and their combination in comparison to 25°C and 100 μmol m(-2)s(-1) under controlled condition. When LC-MS and GC-MS based metabolite profiling was implemented and integrated with targeted RT-qPCR transcript analysis specific responses were observed to the different cues. HL enhanced polyphenol metabolism while HT and its combination with HL induced amino acid and organic acid metabolism with additional effect on polyphenols. The trend of increment in TCA cycle genes like ATCs, ACo1, and IDH in the combined treatment might support the observed increment in organic acids, GABA shunt, and their derivatives. The apparent phenylalanine reduction with polyphenol increment under HL suggests enhanced fueling of the precursor toward the downstream phenylpropanoid pathway. In the polyphenol metabolism, a differential pattern of expression of flavonoid 3',5' hydroxylase and flavonoid 3' hydroxylase was observed under high light (HL) and combined cues which were accompanied by characteristic metabolite profiles. HT decreased glycosylated cyanidin and peonidin forms while the combined cues increased acetylated and coumarylated peonidin forms. Transcription factors regulating anthocyanin metabolism and their methylation, MYB, OMT, UFGT, and DFR, were expressed differentially among the treatments, overall in agreement with the metabolite profiles. Taken together these data provide insights into the coordination of central and secondary metabolism in relation to multiple abiotic stresses.
Keywords: GC–MS; LC–MS; abiotic stress; cell culture; grape; metabolite; transcript.
Figures




References
-
- AbdElgawad H., Peshev D., Zinta G., Ende W., Janssens I., Asard H. (2014). Climate extreme effects on the chemical composition of temperate grassland species under ambient and elevated CO2: a comparison of fructan and non-fructan accumulators. PLoS ONE 9:e92044 10.1371/journal.pone.0092044 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

