Ion-pumping microbial rhodopsins
- PMID: 26442282
- PMCID: PMC4585134
- DOI: 10.3389/fmolb.2015.00052
Ion-pumping microbial rhodopsins
Abstract
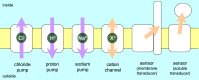
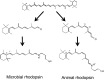
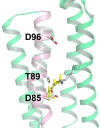
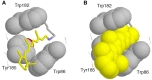
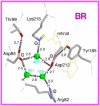
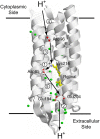
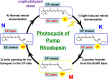
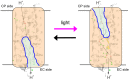
Rhodopsins are light-sensing proteins used in optogenetics. The word "rhodopsin" originates from the Greek words "rhodo" and "opsis," indicating rose and sight, respectively. Although the classical meaning of rhodopsin is the red-colored pigment in our eyes, the modern meaning of rhodopsin encompasses photoactive proteins containing a retinal chromophore in animals and microbes. Animal and microbial rhodopsins possess 11-cis and all-trans retinal, respectively, to capture light in seven transmembrane α-helices, and photoisomerizations into all-trans and 13-cis forms, respectively, initiate each function. Ion-transporting proteins can be found in microbial rhodopsins, such as light-gated channels and light-driven pumps, which are the main tools in optogenetics. Light-driven pumps, such as archaeal H(+) pump bacteriorhodopsin (BR) and Cl(-) pump halorhodopsin (HR), were discovered in the 1970s, and their mechanism has been extensively studied. On the other hand, different kinds of H(+) and Cl(-) pumps have been found in marine bacteria, such as proteorhodopsin (PR) and Fulvimarina pelagi rhodopsin (FR), respectively. In addition, a light-driven Na(+) pump was found, Krokinobacter eikastus rhodopsin 2 (KR2). These light-driven ion-pumping microbial rhodopsins are classified as DTD, TSA, DTE, NTQ, and NDQ rhodopsins for BR, HR, PR, FR, and KR2, respectively. Recent understanding of ion-pumping microbial rhodopsins is reviewed in this paper.
Keywords: H+ transfer; hydrogen bond; light-driven pump; photocycle; photoisomerizatoin; retinal; structural change.
Figures


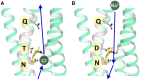
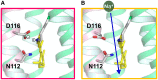
References
-
- Agmon N. (1995). The Grotthuss mechanism. Chem. Phys. Lett. 244, 456–462. 10.1016/0009-2614(95)00905-J - DOI
-
- Balashov S. P., Petrovskaya L. E., Imasheva E. S., Lukashev E. P., Dioumaev A. K., Wang J. M., et al. . (2013). Breaking the carboxyl rule. Lysine96 facilitates protonation of the Schifff base in the photocycle of a retinal protein from Exiguobacterium sibiricum. J. Biol. Chem. 288, 21254–21265. 10.1074/jbc.M113.465138 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

