Mitogen-activated Protein Kinase (MAPK) Activated by Prostaglandin E2 Phosphorylates Connexin 43 and Closes Osteocytic Hemichannels in Response to Continuous Flow Shear Stress
- PMID: 26442583
- PMCID: PMC4653687
- DOI: 10.1074/jbc.M115.683417
Mitogen-activated Protein Kinase (MAPK) Activated by Prostaglandin E2 Phosphorylates Connexin 43 and Closes Osteocytic Hemichannels in Response to Continuous Flow Shear Stress
Abstract
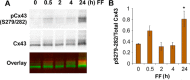
Cx43 hemichannels serve as a portal for the release of prostaglandins, a critical process in mediating biological responses of mechanical loading on bone formation and remodeling. We have previously observed that fluid flow shear stress (FFSS) opens hemichannels; however, sustained FFSS results in hemichannel closure, as continuous opening of hemichannels is detrimental to cell viability and bone remodeling. However, the mechanism that regulates the closure of the hemichannels is unknown. Here, we show that activation of p44/42 ERK upon continuous FFSS leads to Cx43 phosphorylation at Ser(279)-Ser(282), sites known to be phosphorylated sites by p44/42 MAPK. Incubation of osteocytic MLO-Y4 cells with conditioned media (CM) collected after continuous FFSS increased MAPK-dependent phosphorylation of Cx43. CM treatment inhibited hemichannel opening and this inhibition was reversed when cells were pretreated with the MAPK pathway inhibitor. We found that prostaglandin E2 (PGE2) accumulates in the CM in a time-dependent manner. Treatment with PGE2 increased phospho-p44/42 ERK levels and also Cx43 phosphorylation at Ser(279)-Ser(282) sites. Depletion of PGE2 from CM, and pre-treatment with a p44/42 ERK pathway-specific inhibitor, resulted in a complete inhibition of ERK-dependent Cx43 phosphorylation and attenuated the inhibition of hemichannels by CM and PGE2. Consistently, the opening of hemichannels by FFSS was blocked by PGE2 and CM and this blockage was reversed by U0126 and the CM depleted of PGE2. A similar observation was also obtained in isolated primary osteocytes. Together, results from this study suggest that extracellular PGE2 accumulated after continuous FFSS is responsible for activation of p44/42 ERK signaling and subsequently, direct Cx43 phosphorylation by activated ERK leads to hemichannel closure.
Keywords: Hemichannels; Mechanical stimulation; channel activation; connexin; mitogen-activated protein kinase (MAPK); osteocyte; phosphorylation.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






References
-
- Ajubi N. E., Klein-Nulend J., Nijweide P. J., Vrijheid-Lammers T., Alblas M. J., and Burger E. H. (1996) Pulsating fluid flow increases prostaglandin production by cultured chicken osteocytes: a cytoskeleton-dependent process. Biochem. Biophys. Res. Commun. 225, 62–68 - PubMed
-
- Klein-Nulend J., Semeins C. M., Ajubi N. E., Nijweide P. J., and Burger E. H. (1995) Pulsting fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts-correlation with prostaglandin upregulation. Biochem. Biophys. Res. Commun. 217, 640–648 - PubMed
-
- Cherian P. P., Cheng B., Gu S., Sprague E., Bonewald L. F., and Jiang J. X. (2003) Effects of mechanical strain on the function of gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor. J. Biol. Chem. 278, 43146–43156 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

