Dopamine Transporter Activity Is Modulated by α-Synuclein
- PMID: 26442590
- PMCID: PMC4705954
- DOI: 10.1074/jbc.M115.691592
Dopamine Transporter Activity Is Modulated by α-Synuclein
Abstract
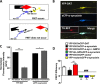
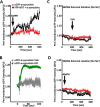
The duration and strength of the dopaminergic signal are regulated by the dopamine transporter (DAT). Drug addiction and neurodegenerative and neuropsychiatric diseases have all been associated with altered DAT activity. The membrane localization and the activity of DAT are regulated by a number of intracellular proteins. α-Synuclein, a protein partner of DAT, is implicated in neurodegenerative disease and drug addiction. Little is known about the regulatory mechanisms of the interaction between DAT and α-synuclein, the cellular location of this interaction, and the functional consequences of this interaction on the basal, amphetamine-induced DAT-mediated dopamine efflux, and membrane microdomain distribution of the transporter. Here, we found that the majority of DAT·α-synuclein protein complexes are found at the plasma membrane of dopaminergic neurons or mammalian cells and that the amphetamine-mediated increase in DAT activity enhances the association of these proteins at the plasma membrane. Further examination of the interaction of DAT and α-synuclein revealed a transient interaction between these two proteins at the plasma membrane. Additionally, we found DAT-induced membrane depolarization enhances plasma membrane localization of α-synuclein, which in turn increases dopamine efflux and enhances DAT localization in cholesterol-rich membrane microdomains.
Keywords: addiction; amphetamine; dopamine; dopamine efflux; dopamine transporter; neurochemistry; synuclein.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






References
-
- Stefansson H., Ophoff R. A., Steinberg S., Andreassen O. A., Cichon S., Rujescu D., Werge T., Pietiläinen O. P., Mors O., Mortensen P. B., Sigurdsson E., Gustafsson O., Nyegaard M., Tuulio-Henriksson A., Ingason A., et al. (2009) Common variants conferring risk of schizophrenia. Nature 460, 744–747 - PMC - PubMed
-
- Varrone A., Marek K. L., Jennings D., Innis R. B., and Seibyl J. P. (2001) [123I]β-CIT SPECT imaging demonstrates reduced density of striatal dopamine transporters in Parkinson's disease and multiple system atrophy. Mov. Disord. 16, 1023–1032 - PubMed
-
- Braak H., Del Tredici K., Rüb U., de Vos R. A., Jansen Steur E. N., and Braak E. (2003) Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging 24, 197–211 - PubMed
-
- Rocha B. A., Fumagalli F., Gainetdinov R. R., Jones S. R., Ator R., Giros B., Miller G. W., and Caron M. G. (1998) Cocaine self-administration in dopamine-transporter knockout mice. Nat. Neurosci. 1, 132–137 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

