GABAergic Signaling within a Limbic-Hypothalamic Circuit Integrates Social and Anxiety-Like Behavior with Stress Reactivity
- PMID: 26442601
- PMCID: PMC4832014
- DOI: 10.1038/npp.2015.311
GABAergic Signaling within a Limbic-Hypothalamic Circuit Integrates Social and Anxiety-Like Behavior with Stress Reactivity
Abstract
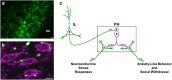
The posterior hypothalamic nucleus (PH) stimulates autonomic stress responses. However, the role of the PH in behavioral correlates of psychiatric illness, such as social and anxiety-like behavior, is largely unexplored, as is the neurochemistry of PH connectivity with limbic and neuroendocrine systems. Thus, the current study tested the hypothesis that GABAergic signaling within the PH is a critical link between forebrain behavior-regulatory nuclei and the neuroendocrine hypothalamus, integrating social and anxiety-related behaviors with physiological stress reactivity. To address this hypothesis, GABAA receptor pharmacology was used to locally inhibit or disinhibit the PH immediately before behavioral measures of social and anxiety-like behavior in rats. Limbic connectivity of the PH was then established by simultaneous co-injection of anterograde and retrograde tracers. Further, the role of PH GABAergic signaling in neuroendocrine stress responses was tested via inhibition/disinhibition of the PH. These studies determined a prominent role for the PH in the expression of anxiety-related behaviors and social withdrawal. Histological analyses revealed divergent stress-activated limbic input to the PH, emanating predominantly from the prefrontal cortex, lateral septum, and amygdala. PH projections also targeted both parvicellular and magnocellular peptidergic neurons in the paraventricular and supraoptic hypothalamus. Further, GABAA receptor pharmacology determined an excitatory effect of the PH on neuroendocrine responses to stress. These data indicate that the PH represents an important stress-integrative center, regulating behavioral processes and connecting the limbic forebrain with neuroendocrine systems. Moreover, the PH appears to be uniquely situated to have a role in stress-related pathologies associated with limbic-hypothalamic dysfunction.
Figures




References
-
- Abrahamson EE, Moore RY (2001). The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res 889: 1–22. - PubMed
-
- Belzung C, Griebel G (2001). Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res 125: 141–149. - PubMed
-
- Bornstein SR, Engeland WC, Ehrhart-Bornstein M, Herman JP (2008). Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metab 19: 175–180. - PubMed
-
- Coolen LM, Wood RI (1998). Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol 399: 189–209. - PubMed
-
- Davis M (1997). Neurobiology of fear responses: the role of the amygdala. J Neuropsychiatry Clin Neurosci 9: 382–402. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

