Stress-Induced Mutagenesis
- PMID: 26442828
- PMCID: PMC4237208
- DOI: 10.1128/ecosalplus.7.2.3
Stress-Induced Mutagenesis
Abstract
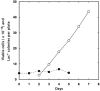
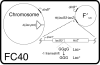
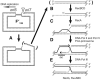
Early research on the origins and mechanisms of mutation led to the establishment of the dogma that, in the absence of external forces, spontaneous mutation rates are constant. However, recent results from a variety of experimental systems suggest that mutation rates can increase in response to selective pressures. This chapter summarizes data demonstrating that,under stressful conditions, Escherichia coli and Salmonella can increase the likelihood of beneficial mutations by modulating their potential for genetic change.Several experimental systems used to study stress-induced mutagenesis are discussed, with special emphasison the Foster-Cairns system for "adaptive mutation" in E. coli and Salmonella. Examples from other model systems are given to illustrate that stress-induced mutagenesis is a natural and general phenomenon that is not confined to enteric bacteria. Finally, some of the controversy in the field of stress-induced mutagenesis is summarized and discussed, and a perspective on the current state of the field is provided.
Figures

References
-
- Aertsen A, Michiels CW. Mrr instigates the SOS response after high pressure stress in Escherichia coli. Mol. Microbiol. 2005;58:1381–91. - PubMed
-
- Aertsen A, Tesfazgi Mebrhatu M, Michiels CW. Activation of the Salmonella typhimurium Mrr protein. Biochem. Biophys. Res. Commun. 2008;367:435–9. - PubMed
-
- Aguirre-Ramírez M, Ramírez-Santos J, Van Melderen L, Gómez-Eichelmann MC. Expression of the F plasmid ccd toxin-antitoxin system in Escherichia coli cells under nutritional stress. Can. J. Microbiol. 2006;52:24–30. - PubMed
-
- Al Mamun AA, Marians KJ, Humayun MZ. DNA polymerase III from Escherichia coli cells expressing mutA mistranslator tRNA is error-prone. J. Biol. Chem. 2002;277:46319–27. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

