DNA Methylation
- PMID: 26442938
- PMCID: PMC4231299
- DOI: 10.1128/ecosalplus.ESP-0003-2013
DNA Methylation
Abstract
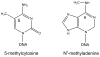
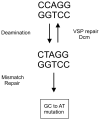
The DNA of Escherichia coli contains 19,120 6-methyladenines and 12,045 5-methylcytosines in addition to the four regular bases, and these are formed by the postreplicative action of three DNA methyltransferases. The majority of the methylated bases are formed by the Dam and Dcm methyltransferases encoded by the dam (DNA adenine methyltransferase) and dcm (DNA cytosine methyltransferase) genes. Although not essential, Dam methylation is important for strand discrimination during the repair of replication errors, controlling the frequency of initiation of chromosome replication at oriC, and the regulation of transcription initiation at promoters containing GATC sequences. In contrast, there is no known function for Dcm methylation, although Dcm recognition sites constitute sequence motifs for Very Short Patch repair of T/G base mismatches. In certain bacteria (e.g., Vibrio cholerae, Caulobacter crescentus) adenine methylation is essential, and, in C. crescentus, it is important for temporal gene expression, which, in turn, is required for coordinating chromosome initiation, replication, and division. In practical terms, Dam and Dcm methylation can inhibit restriction enzyme cleavage, decrease transformation frequency in certain bacteria, and decrease the stability of short direct repeats and are necessary for site-directed mutagenesis and to probe eukaryotic structure and function.
Figures






Similar articles
-
DNA Methylation.EcoSal Plus. 2009 Aug;3(2). doi: 10.1128/ecosalplus.4.4.5. EcoSal Plus. 2009. PMID: 26443769
-
The dam and dcm strains of Escherichia coli--a review.Gene. 1994 May 27;143(1):1-12. doi: 10.1016/0378-1119(94)90597-5. Gene. 1994. PMID: 8200522 Review.
-
Detection of N6-methyladenine in GATC sequences of Selenomonas ruminantium.J Basic Microbiol. 1998;38(4):283-7. J Basic Microbiol. 1998. PMID: 9791949
-
Bacterial DNA methylation and gene transfer efficiency.Biochem Biophys Res Commun. 2000 Oct 5;276(3):1261-4. doi: 10.1006/bbrc.2000.3603. Biochem Biophys Res Commun. 2000. PMID: 11027620
-
[The DNA-methylation state regulates virulence and stress response of Salmonella].C R Biol. 2008 Sep;331(9):648-54. doi: 10.1016/j.crvi.2008.06.002. Epub 2008 Jul 2. C R Biol. 2008. PMID: 18722983 Review. French.
Cited by
-
Sources of artifact in measurements of 6mA and 4mC abundance in eukaryotic genomic DNA.BMC Genomics. 2019 Jun 3;20(1):445. doi: 10.1186/s12864-019-5754-6. BMC Genomics. 2019. PMID: 31159718 Free PMC article.
-
Mapping DNA methylation with high-throughput nanopore sequencing.Nat Methods. 2017 Apr;14(4):411-413. doi: 10.1038/nmeth.4189. Epub 2017 Feb 20. Nat Methods. 2017. PMID: 28218897 Free PMC article.
-
Convergence of DNA methylation and phosphorothioation epigenetics in bacterial genomes.Proc Natl Acad Sci U S A. 2017 Apr 25;114(17):4501-4506. doi: 10.1073/pnas.1702450114. Epub 2017 Apr 11. Proc Natl Acad Sci U S A. 2017. PMID: 28400512 Free PMC article.
-
Genetic and metabolic engineering challenges of C1-gas fermenting acetogenic chassis organisms.FEMS Microbiol Rev. 2021 Mar 16;45(2):fuab008. doi: 10.1093/femsre/fuab008. FEMS Microbiol Rev. 2021. PMID: 33595667 Free PMC article. Review.
-
Synthesizing unmodified, supercoiled circular DNA molecules in vitro.bioRxiv [Preprint]. 2025 Jan 25:2025.01.24.634800. doi: 10.1101/2025.01.24.634800. bioRxiv. 2025. PMID: 39896529 Free PMC article. Preprint.
References
-
- Marinus MG. Methylation of DNA. In: Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE, editors. Escherichia coli and Salmonella: Cellular and Molecular Biology. 2. Vol. 1. ASM Press; Washington, DC: 1996. pp. 782–791.
-
- Løbner-Olesen A, Skovgaard O, Marinus MG. Dam methylation: coordinating cellular processes. Curr Opin Microbiol. 2005;8:154–160. - PubMed
-
- Low DA, Casadesus J. Clocks and switches: bacterial gene regulation by DNA adenine methylation. Curr Opin Microbiol. 2008;11:106–112. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

