Biotin and Lipoic Acid: Synthesis, Attachment, and Regulation
- PMID: 26442940
- PMCID: PMC4233344
- DOI: 10.1128/ecosalplus.ESP-0001-2012
Biotin and Lipoic Acid: Synthesis, Attachment, and Regulation
Abstract
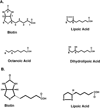
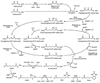
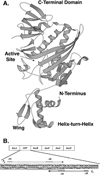
Two vitamins, biotin and lipoic acid, are essential in all three domains of life. Both coenzymes function only when covalently attached to key metabolic enzymes. There they act as "swinging arms" that shuttle intermediates between two active sites (= covalent substrate channeling) of key metabolic enzymes. Although biotin was discovered over 100 years ago and lipoic acid 60 years ago, it was not known how either coenzyme is made until recently. In Escherichia coli the synthetic pathways for both coenzymes have now been worked out for the first time. The late steps of biotin synthesis, those involved in assembling the fused rings, were well described biochemically years ago, although recent progress has been made on the BioB reaction, the last step of the pathway in which the biotin sulfur moiety is inserted. In contrast, the early steps of biotin synthesis, assembly of the fatty acid-like "arm" of biotin were unknown. It has now been demonstrated that the arm is made by using disguised substrates to gain entry into the fatty acid synthesis pathway followed by removal of the disguise when the proper chain length is attained. The BioC methyltransferase is responsible for introducing the disguise, and the BioH esterase is responsible for its removal. In contrast to biotin, which is attached to its cognate proteins as a finished molecule, lipoic acid is assembled on its cognate proteins. An octanoyl moiety is transferred from the octanoyl acyl carrier protein of fatty acid synthesis to a specific lysine residue of a cognate protein by the LipB octanoyltransferase followed by sulfur insertion at carbons C-6 and C-8 by the LipA lipoyl synthetase. Assembly on the cognate proteins regulates the amount of lipoic acid synthesized, and, thus, there is no transcriptional control of the synthetic genes. In contrast, transcriptional control of the biotin synthetic genes is wielded by a remarkably sophisticated, yet simple, system, exerted through BirA, a dual-function protein that both represses biotin operon transcription and ligates biotin to its cognate proteins.
Figures

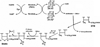




Similar articles
-
Biotin and Lipoic Acid: Synthesis, Attachment, and Regulation.EcoSal Plus. 2008 Sep;3(1). doi: 10.1128/ecosalplus.3.6.3.5. EcoSal Plus. 2008. PMID: 26443737
-
Protein-protein interactions in assembly of lipoic acid on the 2-oxoacid dehydrogenases of aerobic metabolism.J Biol Chem. 2011 Mar 11;286(10):8263-8276. doi: 10.1074/jbc.M110.194191. Epub 2011 Jan 5. J Biol Chem. 2011. PMID: 21209092 Free PMC article.
-
Lipoic acid synthesis: a new family of octanoyltransferases generally annotated as lipoate protein ligases.Biochemistry. 2010 Nov 23;49(46):10024-36. doi: 10.1021/bi101215f. Epub 2010 Oct 27. Biochemistry. 2010. PMID: 20882995 Free PMC article.
-
Closing in on complete pathways of biotin biosynthesis.Mol Biosyst. 2011 Jun;7(6):1811-21. doi: 10.1039/c1mb05022b. Epub 2011 Mar 24. Mol Biosyst. 2011. PMID: 21437340 Review.
-
Assembly of Lipoic Acid on Its Cognate Enzymes: an Extraordinary and Essential Biosynthetic Pathway.Microbiol Mol Biol Rev. 2016 Apr 13;80(2):429-50. doi: 10.1128/MMBR.00073-15. Print 2016 Jun. Microbiol Mol Biol Rev. 2016. PMID: 27074917 Free PMC article. Review.
Cited by
-
Active and inactive forms of biotin synthase occur in Heterodera glycines.J Nematol. 2019 Oct 25;51:e2019-69. doi: 10.21307/jofnem-2019-069. eCollection 2019. J Nematol. 2019. PMID: 34179812 Free PMC article.
-
Exploring the microbial communities in coastal cenote and their hidden biotechnological potential.Microb Genom. 2025 Apr;11(4):001382. doi: 10.1099/mgen.0.001382. Microb Genom. 2025. PMID: 40178526 Free PMC article.
-
Transcriptional Repression of the VC2105 Protein by Vibrio FadR Suggests that It Is a New Auxiliary Member of the fad Regulon.Appl Environ Microbiol. 2016 Apr 18;82(9):2819-2832. doi: 10.1128/AEM.00293-16. Print 2016 May. Appl Environ Microbiol. 2016. PMID: 26944841 Free PMC article.
-
PdhR, the pyruvate dehydrogenase repressor, does not regulate lipoic acid synthesis.Res Microbiol. 2014 Jul-Aug;165(6):429-38. doi: 10.1016/j.resmic.2014.04.005. Epub 2014 May 9. Res Microbiol. 2014. PMID: 24816490 Free PMC article.
-
A Novel Lipoate-Protein Ligase, Mhp-LplJ, Is Required for Lipoic Acid Metabolism in Mycoplasma hyopneumoniae.Front Microbiol. 2021 Jan 18;11:631433. doi: 10.3389/fmicb.2020.631433. eCollection 2020. Front Microbiol. 2021. PMID: 33584596 Free PMC article.
References
-
- Visser CM, Kellogg RM. Biotin. Its place in evolution. J Mol Evol. 1978;11:171–187. - PubMed
-
- Green NM. Avidin. Adv Protein Chem. 1975;29:85–133. - PubMed
-
- Wifling K, Dimroth P. Isolation and characterization of oxaloacetate decarboxylase of I, a sodium ion pump. Arch Microbiol. 1989;152:584–588. - PubMed
-
- Woehlke G, Dimroth P. Anaerobic growth of Salmonella typhimurium on l(+)- and d(−)-tartrate involves an oxaloacetate decarboxylase Na+ pump. Arch Microbiol. 1994;162:233–237. - PubMed
-
- Woehlke G, Wifling K, Dimroth P. Sequence of the sodium ion pump oxaloacetate decarboxylase from Salmonella typhimurium. J Biol Chem. 1992;267:22798–22803. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

