Resting state of the human proton channel dimer in a lipid bilayer
- PMID: 26443860
- PMCID: PMC4640771
- DOI: 10.1073/pnas.1515043112
Resting state of the human proton channel dimer in a lipid bilayer
Abstract
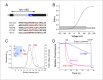
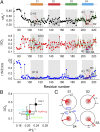
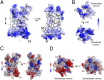
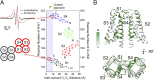
The voltage-gated proton channel Hv1 plays a critical role in the fast proton translocation that underlies a wide range of physiological functions, including the phagocytic respiratory burst, sperm motility, apoptosis, and metastatic cancer. Both voltage activation and proton conduction are carried out by a voltage-sensing domain (VSD) with strong similarity to canonical VSDs in voltage-dependent cation channels and enzymes. We set out to determine the structural properties of membrane-reconstituted human proton channel (hHv1) in its resting conformation using electron paramagnetic resonance spectroscopy together with biochemical and computational methods. We evaluated existing structural templates and generated a spectroscopically constrained model of the hHv1 dimer based on the Ci-VSD structure at resting state. Mapped accessibility data revealed deep water penetration through hHv1, suggesting a highly focused electric field, comprising two turns of helix along the fourth transmembrane segment. This region likely contains the H(+) selectivity filter and the conduction pore. Our 3D model offers plausible explanations for existing electrophysiological and biochemical data, offering an explicit mechanism for voltage activation based on a one-click sliding helix conformational rearrangement.
Keywords: EPR; MD simulation; proton channel; voltage sensing domain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Structural revelations of the human proton channel.Proc Natl Acad Sci U S A. 2015 Nov 3;112(44):13430-1. doi: 10.1073/pnas.1518486112. Epub 2015 Oct 14. Proc Natl Acad Sci U S A. 2015. PMID: 26466610 Free PMC article. No abstract available.
References
-
- Sasaki M, Takagi M, Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312(5773):589–592. - PubMed
-
- DeCoursey TE, Morgan D, Cherny VV. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. 2003;422(6931):531–534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

