Src inhibition blocks renal interstitial fibroblast activation and ameliorates renal fibrosis
- PMID: 26444028
- PMCID: PMC4824676
- DOI: 10.1038/ki.2015.293
Src inhibition blocks renal interstitial fibroblast activation and ameliorates renal fibrosis
Expression of concern in
-
Yan Y, Ma L, Zhou X, et al. Src inhibition blocks renal interstitial fibroblast activation and ameliorates renal fibrosis. Kidney Int. 2016;89:68-81.Kidney Int. 2017 Sep;92(3):770-772. doi: 10.1016/j.kint.2017.06.007. Kidney Int. 2017. PMID: 28807271 No abstract available.
Abstract
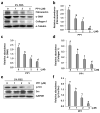
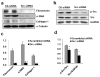
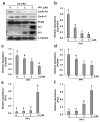
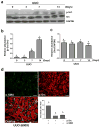
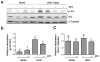
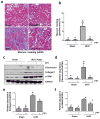
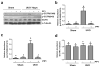
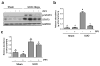
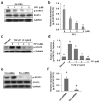
Increased Src activity has been associated with the pathogenesis of renal tumors and some glomerular diseases, but its role in renal interstitial fibrosis remains elusive. To evaluate this, cultured renal interstitial fibroblasts (NRK-49F) were treated with PP1, a selective inhibitor of Src. This resulted in decreased expression of α-smooth muscle actin, fibronectin, and collagen I in response to serum, angiotension II, or transforming growth factor-β1 (TGF-β1). Silencing Src with siRNA also inhibited expression of those proteins. Furthermore, inhibition of Src activity blocked renal fibroblast proliferation. In a murine model of renal interstitial fibrosis induced by unilateral ureteral obstruction, the active form of Src (phopsho-Src Tyr416) was upregulated in both renal interstitial fibroblasts and renal tubular cells of the fibrotic kidney. Its inactivation reduced renal fibroblast activation and attenuated extracellular matrix protein deposition. Src inhibition also suppressed activation of TGF-β1 signaling, activation of the epidermal growth factor receptor and STAT3, and reduced the number of renal epithelial cells arrested at the G2/M phase of the cell cycle after ureteral obstruction. Thus, Src is an important mediator of renal interstitial fibroblast activation and renal fibrosis, and we suggest that Src is a potential therapeutic target for treatment of chronic renal fibrosis.
Keywords: Src; epidermal growth factor receptor; renal fibrogenesis; renal interstitial fibroblasts; transforming growth factor-β1; α-smooth muscle actin.
Copyright © 2015 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Liu Y. Rapamycin and chronic kidney disease: beyond the inhibition of inflammation. Kidney Int. 2006;69:1925–1927. - PubMed
-
- Ruggenenti P, Cravedi P, Remuzzi G. Mechanisms and treatment of CKD. J Am Soc Nephrol. 2012;23:1917–1928. - PubMed
-
- Samarakoon R, Dobberfuhl AD, Cooley C, et al. Induction of renal fibrotic genes by TGF-beta1 requires EGFR activation, p53 and reactive oxygen species. Cell Signal. 2013;25:2198–2209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

