Therapeutic Use of Soluble Fas Ligand Ameliorates Acute and Recurrent Herpetic Stromal Keratitis in Mice
- PMID: 26444718
- PMCID: PMC4594530
- DOI: 10.1167/iovs.15-16588
Therapeutic Use of Soluble Fas Ligand Ameliorates Acute and Recurrent Herpetic Stromal Keratitis in Mice
Abstract
Purpose: The present study was designed to test the therapeutic value of soluble FasL (sFasL) in an acute model of herpetic stromal keratitis (HSK) and, more importantly, a recurrent model of HSK using BALB/c, BALB-lpr, and National Institutes of Health (NIH) mice.
Methods: Mice were infected either acutely with the KOS strain of herpes simplex virus 1 (HSV-1) or latently with the McKrae strain of HSV-1. Acutely infected mice as well as ultraviolet-B (UV-B) reactivated mice (recurrent infection) were treated with sFasL, or soluble TNF-related apoptosis inducing ligand (sTRAIL), or BSA daily or 3 times/wk by using either a combination of subconjunctival injection and topical ointment, or with topical ointment alone. These mice then were evaluated for corneal opacity and neovascularization for 6 weeks.
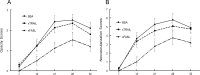
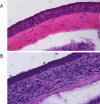
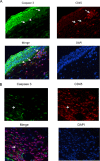
Results: Following acute and recurrent HSV-1 infection, wild-type BALB/c mice treated with sFasL displayed significantly reduced incidence of corneal opacity and neovascularization compared to the control animals. However, BALB-lpr mice, which are deficient in Fas+ inflammatory cells, displayed no such differences in ocular disease, as expected. Latently infected NIH mice treated with sFasL displayed similar results. Flow cytometric analysis revealed that the corneal inflammatory infiltrate in those treated with sFasL was significantly less than in sTRAIL- or BSA-treated mice. Furthermore, corneas from sFasL-treated mice displayed relatively more cells undergoing apoptosis.
Conclusions: This study provides evidence that sFasL treatment has potential therapeutic benefit in reducing inflammatory infiltrate and neovascularization in primary and recurrent forms of HSK, and that it does so by augmenting the restriction of Fas+ inflammatory cells mediated by membrane FasL.
Figures




 20.
20.

Similar articles
-
Effect of Fas and Fas ligand deficiency in resistance of C57BL/6 mice to HSV-1 keratitis and chorioretinitis.Invest Ophthalmol Vis Sci. 2001 Oct;42(11):2505-9. Invest Ophthalmol Vis Sci. 2001. PMID: 11581190
-
[Cytokine expression in murine cornea tissue during recurrent herpetic stromal keratitis].Zhonghua Yan Ke Za Zhi. 2005 May;41(5):403-8. Zhonghua Yan Ke Za Zhi. 2005. PMID: 15938803 Chinese.
-
IL-1 and TNF-alpha are important factors in the pathogenesis of murine recurrent herpetic stromal keratitis.Invest Ophthalmol Vis Sci. 2000 Jan;41(1):96-102. Invest Ophthalmol Vis Sci. 2000. PMID: 10634607
-
Management of herpes simplex virus stromal keratitis: an evidence-based review.Surv Ophthalmol. 2009 Mar-Apr;54(2):226-34. doi: 10.1016/j.survophthal.2008.12.004. Surv Ophthalmol. 2009. PMID: 19298901 Review.
-
Corneal lymphangiogenesis in herpetic stromal keratitis.Surv Ophthalmol. 2015 Jan-Feb;60(1):60-71. doi: 10.1016/j.survophthal.2014.06.001. Epub 2014 Jun 10. Surv Ophthalmol. 2015. PMID: 25444520 Free PMC article. Review.
Cited by
-
Kinetic Analysis of Biomarkers in a Cohort of US Patients With Ebola Virus Disease.Clin Infect Dis. 2016 Aug 15;63(4):460-7. doi: 10.1093/cid/ciw334. Epub 2016 Jun 27. Clin Infect Dis. 2016. PMID: 27353663 Free PMC article.
-
Pathological processes activated by herpes simplex virus-1 (HSV-1) infection in the cornea.Cell Mol Life Sci. 2019 Feb;76(3):405-419. doi: 10.1007/s00018-018-2938-1. Epub 2018 Oct 16. Cell Mol Life Sci. 2019. PMID: 30327839 Free PMC article. Review.
-
A Potential of sFasL in Preventing Gland Injury in Sjogren's Syndrome.Biomed Res Int. 2017;2017:5981432. doi: 10.1155/2017/5981432. Epub 2017 Feb 23. Biomed Res Int. 2017. PMID: 28326325 Free PMC article.
-
MicroRNAs Expression in Response to rhNGF in Epithelial Corneal Cells: Focus on Neurotrophin Signaling Pathway.Int J Mol Sci. 2022 Mar 25;23(7):3597. doi: 10.3390/ijms23073597. Int J Mol Sci. 2022. PMID: 35408969 Free PMC article.
-
Impact of Type I Interferon on the Safety and Immunogenicity of an Experimental Live-Attenuated Herpes Simplex Virus 1 Vaccine in Mice.J Virol. 2017 Mar 13;91(7):e02342-16. doi: 10.1128/JVI.02342-16. Print 2017 Apr 1. J Virol. 2017. PMID: 28122977 Free PMC article.
References
-
- Pepose JS,, Leib DA,, Stuart PM,, Easty EL. Herpes simplex virus diseases: anterior segment of the eye. Pepose JS,, Holland GAN,, Wilhelmus KR, Ocular Infection and Immunity. St. Louis, MO: Mosby; 1996: 905–932.
-
- Thomas J,, Gangappa S,, Kanangat S,, Rouse BT. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stromal keratitis. J Immunol. 1997; 158: 1383–1391. - PubMed
-
- Maertzdorf J,, Verjans GM,, Remeijer L,, van der Kooi A,, Osterhaus AD. Restricted T cell receptor beta-chain variable region protein use by cornea-derived CD4+ and CD8+ herpes simplex virus-specific T cells in patients with herpetic stromal keratitis. J Infect Dis. 2003; 187: 550–558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

