Towards understanding the de-adoption of low-value clinical practices: a scoping review
- PMID: 26444862
- PMCID: PMC4596285
- DOI: 10.1186/s12916-015-0488-z
Towards understanding the de-adoption of low-value clinical practices: a scoping review
Abstract
Background: Low-value clinical practices are common in healthcare, yet the optimal approach to de-adopting these practices is unknown. The objective of this study was to systematically review the literature on de-adoption, document current terminology and frameworks, map the literature to a proposed framework, identify gaps in our understanding of de-adoption, and identify opportunities for additional research.
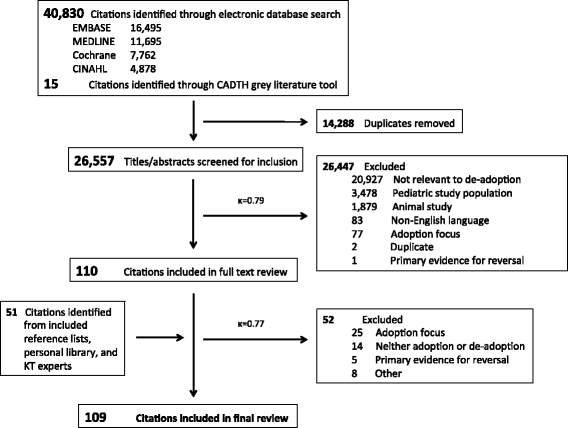
Methods: MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, the Cochrane Database of Abstracts and Reviews of Effects, and CINAHL Plus were searched from 1 January 1990 to 5 March 2014. Additional citations were identified from bibliographies of included citations, relevant websites, the PubMed 'related articles' function, and contacting experts in implementation science. English-language citations that referred to de-adoption of clinical practices in adults with medical, surgical, or psychiatric illnesses were included. Citation selection and data extraction were performed independently and in duplicate.
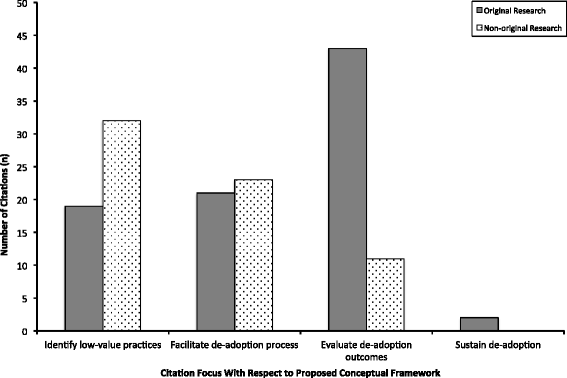
Results: From 26,608 citations, 109 were included in the final review. Most citations (65%) were original research with the majority (59%) published since 2010. There were 43 unique terms referring to the process of de-adoption-the most frequently cited was "disinvest" (39% of citations). The focus of most citations was evaluating the outcomes of de-adoption (50%), followed by identifying low-value practices (47%), and/or facilitating de-adoption (40%). The prevalence of low-value practices ranged from 16% to 46%, with two studies each identifying more than 100 low-value practices. Most articles cited randomized clinical trials (41%) that demonstrate harm (73%) and/or lack of efficacy (63%) as the reason to de-adopt an existing clinical practice. Eleven citations described 13 frameworks to guide the de-adoption process, from which we developed a model for facilitating de-adoption. Active change interventions were associated with the greatest likelihood of de-adoption.
Conclusions: This review identified a large body of literature that describes current approaches and challenges to de-adoption of low-value clinical practices. Additional research is needed to determine an ideal strategy for identifying low-value practices, and facilitating and sustaining de-adoption. In the meantime, this study proposes a model that providers and decision-makers can use to guide efforts to de-adopt ineffective and harmful practices.
Figures


Comment in
-
De-adoption and its 43 related terms: harmonizing low-value care terminology.BMC Med. 2015 Oct 20;13:273. doi: 10.1186/s12916-015-0511-4. BMC Med. 2015. PMID: 26486727 Free PMC article.
References
-
- Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators. Van De Werf F, Adgey J, Ardissino D, Armstrong PW, Aylward P, et al. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. 1999;354:716–722. doi: 10.1016/S0140-6736(99)07403-6. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

