Protocol for a randomised pragmatic policy trial of nicotine products for quitting or long-term substitution in smokers
- PMID: 26444980
- PMCID: PMC4596390
- DOI: 10.1186/s12889-015-2366-1
Protocol for a randomised pragmatic policy trial of nicotine products for quitting or long-term substitution in smokers
Abstract
Background: Smoking is Australia's leading preventable cause of premature mortality and a major contributor to the national disease burden. If quit rates do not dramatically improve, then smoking will continue to be a major public health issue for decades to come. Harm-reduction approaches using novel nicotine products like e-cigarettes as long term replacements for smoking have the potential to improve quit rates. However, little research has assessed such approaches.
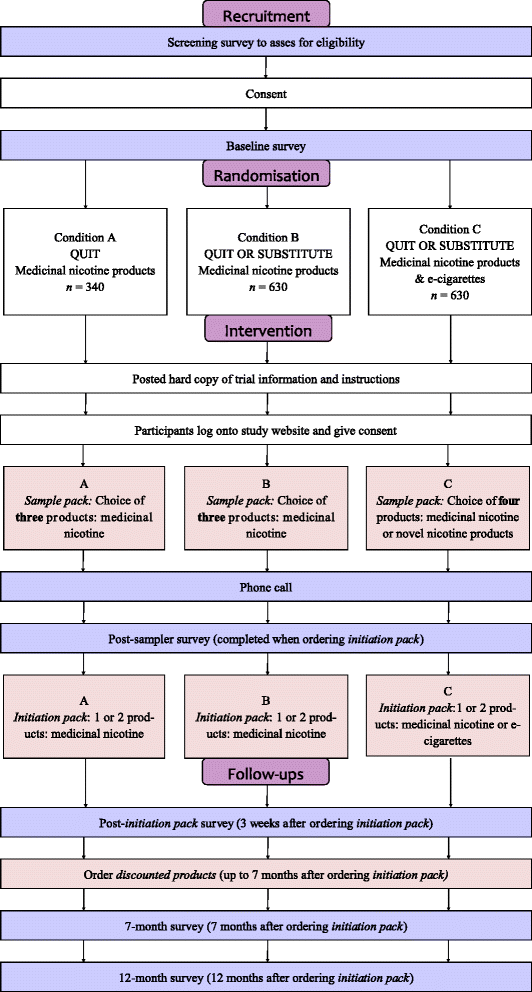
Design: Three-arm parallel-group pragmatic randomised controlled trial.
Participants: People living in Australia who are at least 18 years old, smoke five or more cigarettes per day and are willing to try a sample of nicotine products.
Intervention: Participants are randomised to receive standard quit advice and medicinal nicotine (Condition A); quit or substitute advice and medicinal nicotine (Condition B); or quit or substitute advice and medicinal nicotine and e-cigarettes (Condition C). Participants choose which (if any) nicotine products to receive to try in a free sample pack followed by a two to three week free supply of their favourite product(s) and the option to purchase more at a discounted price. Follow-up surveys will assess nicotine product use and smoking.
Primary outcome: Continuous abstinence for at least 6 months. Target sample size: 1600 people (Condition A: 340; Condition B: 630; Condition C: 630) provides at least 80 % power at p = 0.05 to detect a 5 % difference in abstinence rates between each condition.
Discussion: This trial will provide data on tobacco harm-reduction approaches and in particular the use of e-cigarettes as a replacement for smoking.
Trial registration: Australian and New Zealand Clinical Trials Registry: ACTRN12612001210864. Date of registration: 15/11/2012.
Figures
References
-
- Begg SJ, Vos T, Barker B, Stanley L, Lopez AD. Burden of disease and injury in Australia in the new millennium: measuring health loss from diseases, injuries and risk factors. Med J Aust. 2008;188(1):36. - PubMed
-
- Tobacco Working Group . Technical report no. 2. Tobacco control in Australia: making smoking history. Canberra: National Preventative Health Taskforce; 2008.
-
- Royal College of Physicians . Harm reduction in nicotine addiction: helping people who can’t quit. London: Tobacco Advisory Group of the Royal College of Physicians; 2007.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical