Cysteine-Rich Atrial Secretory Protein from the Snail Achatina achatina: Purification and Structural Characterization
- PMID: 26444993
- PMCID: PMC4596865
- DOI: 10.1371/journal.pone.0138787
Cysteine-Rich Atrial Secretory Protein from the Snail Achatina achatina: Purification and Structural Characterization
Abstract
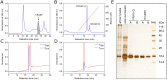
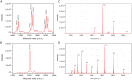
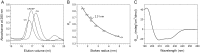
Despite extensive studies of cardiac bioactive peptides and their functions in molluscs, soluble proteins expressed in the heart and secreted into the circulation have not yet been reported. In this study, we describe an 18.1-kDa, cysteine-rich atrial secretory protein (CRASP) isolated from the terrestrial snail Achatina achatina that has no detectable sequence similarity to any known protein or nucleotide sequence. CRASP is an acidic, 158-residue, N-glycosylated protein composed of eight alpha-helical segments stabilized with five disulphide bonds. A combination of fold recognition algorithms and ab initio folding predicted that CRASP adopts an all-alpha, right-handed superhelical fold. CRASP is most strongly expressed in the atrium in secretory atrial granular cells, and substantial amounts of CRASP are released from the heart upon nerve stimulation. CRASP is detected in the haemolymph of intact animals at nanomolar concentrations. CRASP is the first secretory protein expressed in molluscan atrium to be reported. We propose that CRASP is an example of a taxonomically restricted gene that might be responsible for adaptations specific for terrestrial pulmonates.
Conflict of interest statement
Figures
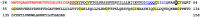



Similar articles
-
Atrial granular cells of the snail Achatina fulica release proteins into hemolymph after stimulation of the heart nerve.J Exp Biol. 2009 Oct;212(Pt 20):3211-20. doi: 10.1242/jeb.029108. J Exp Biol. 2009. PMID: 19801425
-
Nerve-granular cell communication in the atrium of the snail Achatina achatina occurs via the cardioexcitatory transmitters serotonin and FMRFamide.Cell Tissue Res. 2016 Nov;366(2):245-254. doi: 10.1007/s00441-016-2483-x. Epub 2016 Sep 23. Cell Tissue Res. 2016. PMID: 27660155
-
Hsp70 in the atrial neuroendocrine units of the snail, Achatina fulica.Cell Biol Int. 2007 Apr;31(4):413-9. doi: 10.1016/j.cellbi.2007.01.027. Epub 2007 Jan 21. Cell Biol Int. 2007. PMID: 17353135
-
Identifiable Achatina giant neurones: their localizations in ganglia, axonal pathways and pharmacological features.Gen Pharmacol. 1996 Jan;27(1):3-32. doi: 10.1016/0306-3623(95)00113-1. Gen Pharmacol. 1996. PMID: 8742492 Review.
-
Comparative aspects of structure and action of molluscan neuropeptides.Experientia. 1992 May 15;48(5):448-56. doi: 10.1007/BF01928163. Experientia. 1992. PMID: 1601109 Review.
Cited by
-
Chemical synthesis of Torenia plant pollen tube attractant proteins by KAHA ligation.RSC Chem Biol. 2022 Mar 18;3(6):721-727. doi: 10.1039/d2cb00039c. eCollection 2022 Jun 8. RSC Chem Biol. 2022. PMID: 35755195 Free PMC article.
-
Edible Snail Production in Europe.Animals (Basel). 2022 Oct 11;12(20):2732. doi: 10.3390/ani12202732. Animals (Basel). 2022. PMID: 36290118 Free PMC article. Review.
References
-
- J H.D.. Circulatory systems of gastropods and bivalves In: Saleuddin ASM, Wilbur KM, editors. The Mollusca. New York: Academic Press; 1983. p. 189–238.
-
- Cottrell GA, Osborne N. A neurosecretory system terminating in the Helix heart. Comp Biochem Physiol. 1969. March;28(3):1455–9.
-
- De With ND, van der Schors RC, Boer HH, Ebberink RHM. The sodium influx stimulating peptide of the pulmonate freshwater snail Lymnaea stagnalis. Peptides. 1993. July;14(4):783–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

