New all oral therapy for chronic hepatitis C virus (HCV): a novel long-term cost comparison
- PMID: 26445564
- PMCID: PMC4595188
- DOI: 10.1186/s12962-015-0043-y
New all oral therapy for chronic hepatitis C virus (HCV): a novel long-term cost comparison
Abstract
Background: In the US, the prevalence of hepatitis C virus (HCV) has surpassed the prevalence of human immunodeficiency virus (HIV), with about 3.3 million people chronically infected with the disease. Given the aging of the Baby Boomer generation and the subsequent implementation of age-based screening recommendations, HCV diagnoses are expected to increase. Utilization of anti-viral pharmacotherapy is also expected to increase as more effective and tolerable all-oral therapies for HCV become available in the United States. This research allows payors to assess the disease burden and treatment impact of HCV in their member group.
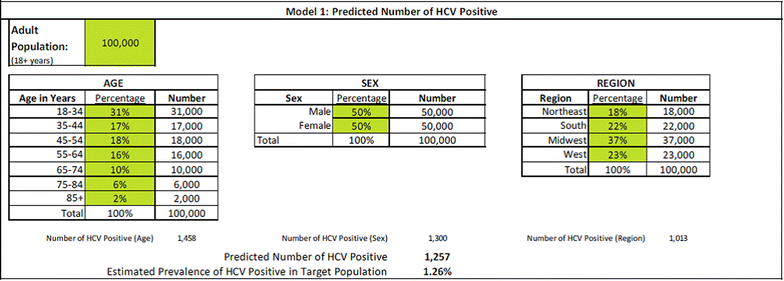
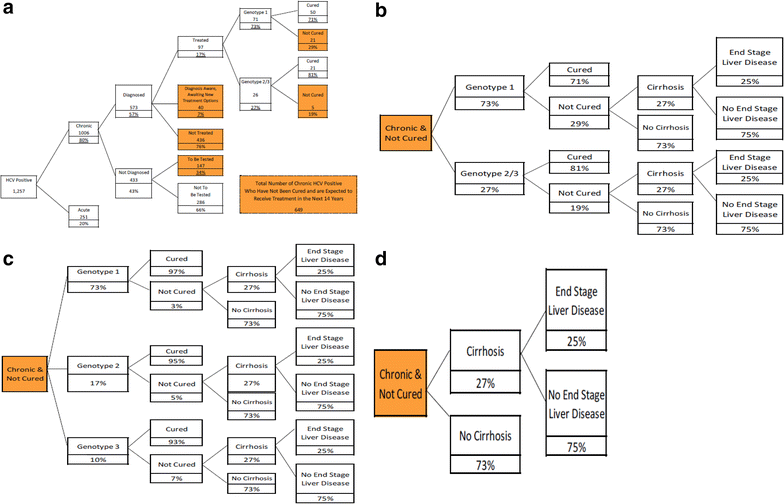
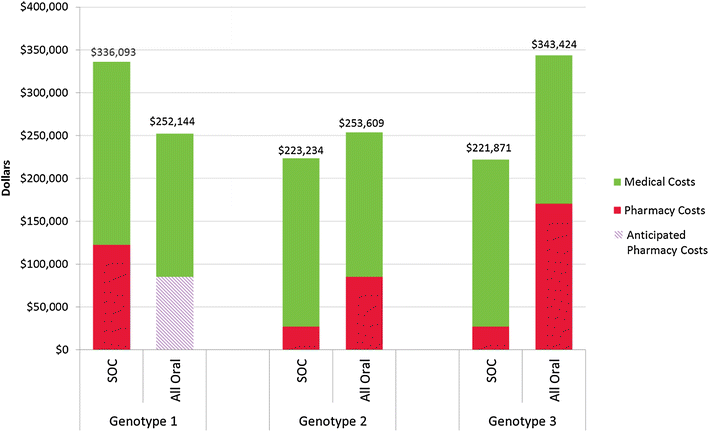
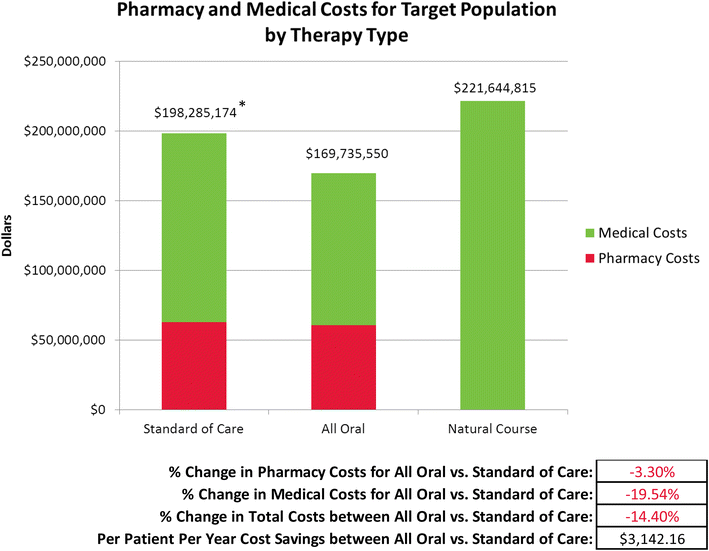
Methods: A set of three integrated economic models was developed to estimate the disease and cost burden of HCV based on existing literature, wholesale acquisition costs, industry standards, and actuarial judgment. Model 1 estimates the HCV antibody prevalence of HCV in a payer's member group based on population size and the age, sex, and region distribution of the members. Model 2 predicts the number of uncured chronic HCV members who represent the future treatment and medical cost burden for the payer over the next 14 years. Model 3 contrasts the pharmacy, medical, and overall costs for treatment and medical care over 14 years for three therapeutic scenarios: interferon-based standard of care (SOC), all oral therapy, and natural course of disease progression, while accounting for the frequency of HCV genotype within the member population.
Results: In a payer population of 100,000 members with an age, sex, and region distribution matching the United States, the seroprevalence of HCV was estimated to be 1.26 %. Combined pharmacy and medical costs for uncured chronic HCV positive members was least expensive for all oral therapy. The per patient with HCV cost savings for all oral therapy compared to SOC were about $3000 per year over 14 years. In a sensitivity analysis, the 12-week all oral therapy for genotype 1 provided overall cost savings vs. a 24-week interferon-based SOC regimen until all oral therapy costs exceeded $99,000.
Conclusions: In most modeled scenarios, the all-oral therapeutic scenario was less costly than SOC, even in sensitivity analyses.
Keywords: All oral therapy; Antiviral agents; Cost-savings analysis; Economic modeling; Hepatitis c prevalence; Hepatitis c treatment; Hepatitis c virus; Interferon; Managed care.
Figures
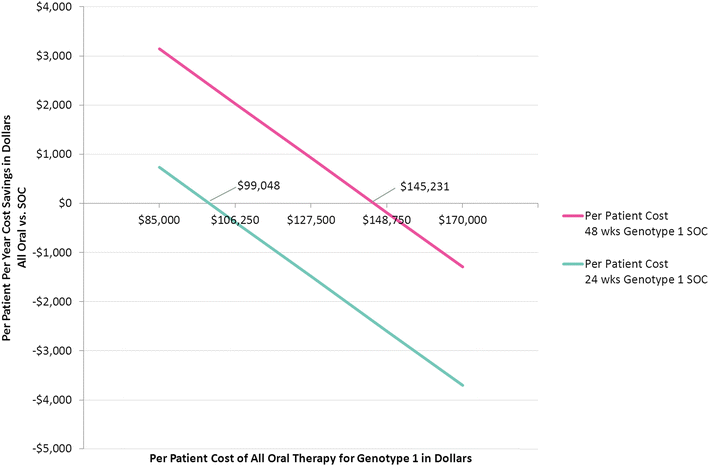
References
-
- Sexually transmitted diseases treatment guidelines. 2010. [http://www.cdc.gov/std/treatment/2010/hepC.htm]. Accessed 19 Sep 2013.
-
- Hepatitis C FAQs for Health Professionals. [http://www.cdc.gov/hepatitis/HCV/HCVfaq.htm]. Accessed 19 Sep 2013.
-
- Moyer VA, U.S. Preventive Services Task Force Screening for hepatitis C virus infection in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2013;159:348–357. - PubMed
-
- Blatt LM, Mutchnick MG, Tong MJ, Klion FM, Lebovics E, Freilich B, Bach N, Smith C, Herrera J, Tobias H, et al. Assessment of hepatitis C virus RNA and genotype from 6807 patients with chronic hepatitis C in the United States. J Viral Hepat. 2000;7:196–202. doi: 10.1046/j.1365-2893.2000.00221.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

