FSP1(+) fibroblast subpopulation is essential for the maintenance and regeneration of medullary thymic epithelial cells
- PMID: 26445893
- PMCID: PMC4597222
- DOI: 10.1038/srep14871
FSP1(+) fibroblast subpopulation is essential for the maintenance and regeneration of medullary thymic epithelial cells
Abstract
Thymic epithelial cells (TECs) form a 3-dimentional network supporting thymocyte development and maturation. Besides epithelium and thymocytes, heterogeneous fibroblasts are essential components in maintaining thymic microenvironments. However, thymic fibroblast characteristics, development and function remain to be determined. We herein found that thymic non-hematopoietic CD45(-)FSP1(+) cells represent a unique Fibroblast specific protein 1 (FSP1)(-)fibroblast-derived cell subset. Deletion of these cells in FSP1-TK transgenic mice caused thymus atrophy due to the loss of TECs, especially mature medullary TECs (MHCII(high), CD80(+) and Aire(+)). In a cyclophosphamide-induced thymus injury and regeneration model, lack of non-hematopoietic CD45(-)FSP1(+) fibroblast subpopulation significantly delayed thymus regeneration. In fact, thymic FSP1(+) fibroblasts released more IL-6, FGF7 and FSP1 in the culture medium than their FSP1(-) counterparts. Further experiments showed that the FSP1 protein could directly enhance the proliferation and maturation of TECs in the in vitro culture systems. FSP1 knockout mice had significantly smaller thymus size and less TECs than their control. Collectively, our studies reveal that thymic CD45(-)FSP1(+) cells are a subpopulation of fibroblasts, which is crucial for the maintenance and regeneration of TECs especially medullary TECs through providing IL-6, FGF7 and FSP1.
Figures



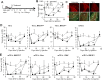



References
-
- Rodewald H. R. Thymus organogenesis. Annu Rev Immunol. 26, 355–388 (2008). - PubMed
-
- Manley N. R., Richie E. R., Blackburn C. C., Condie B. G. & Sage J. Structure and function of the thymic microenvironment. Front Biosci (Landmark Ed). 16, 2461–2477 (2011). - PubMed
-
- Anderson G. & Takahama Y. Thymic epithelial cells: working class heroes for T cell development and repertoire selection. Trends Immunol. 33, 256–263 (2012). - PubMed
-
- Anderson G., Lane P. J. & Jenkinson E. J. Generating intrathymic microenvironments to establish T-cell tolerance. Nat Rev Immunol. 7, 954–963 (2007). - PubMed
-
- Manley N. R. & Condie B. G. Transcriptional regulation of thymus organogenesis and thymic epithelial cell differentiation. Prog Mol Biol Transl Sci. 92, 103–120 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

