SjCa8, a calcium-binding protein from Schistosoma japonicum, inhibits cell migration and suppresses nitric oxide release of RAW264.7 macrophages
- PMID: 26445908
- PMCID: PMC4597762
- DOI: 10.1186/s13071-015-1119-4
SjCa8, a calcium-binding protein from Schistosoma japonicum, inhibits cell migration and suppresses nitric oxide release of RAW264.7 macrophages
Abstract
Background: Schistosomiasis is considered second only to malaria as the most devastating parasitic disease in tropical countries. Schistosome cercariae invade the host by penetrating the skin and migrate though the lungs and portal circulation to their final destination in the hepatic portal system and eventually the mesenteric veins. Previous studies have shown that the cytotoxic pathways that target schistosomulum in the lung-stage involve nitric oxide (NO) produced by macrophages. By contrast, skin-stage schistosomulas can evade clearance, indicating that they might be freed from macrophage NO-mediated cytotoxicity to achieve immune evasion; however, the critical molecules and mechanisms involved remain unknown.
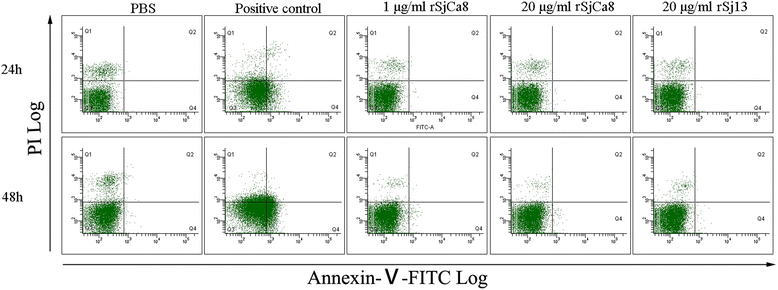
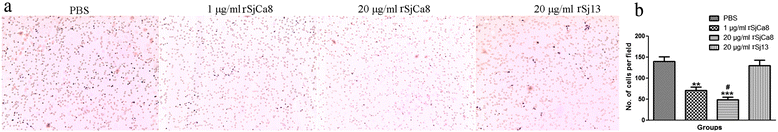
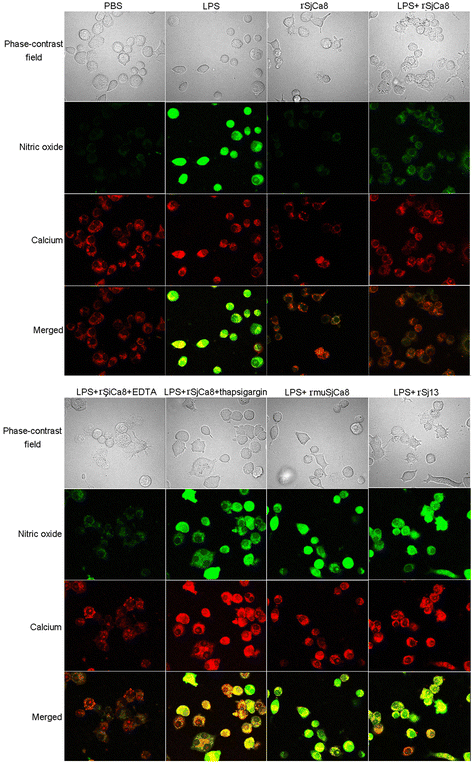
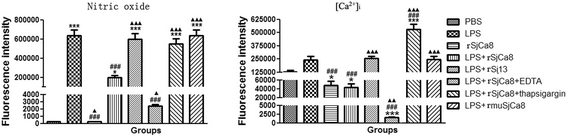
Methods: Recombinant SjCa8 (rSjCa8), an 8-kDa calcium-binding protein that is stage-specifically expressed in cercaria and early skin-stage schistosomulas of Schistosoma japonicum, was incubated with mouse RAW264.7 macrophages. Effects on macrophage proliferation were determined using Cell Counting Kit-8. Next, transwell assay was carried out to further investigate the role of rSjCa8 in macrophage migration. The effects of rSjCa8 on macrophage apoptosis were evaluated using confocal microscopy and flow cytometry. Additional impacts of rSjCa8 on NO release by lipopolysaccharide (LPS)-stimulated macrophages as well as the underlying mechanisms were explored using fluorescent probe, nitric oxide signaling pathway microarray, quantitative real-time PCR, mutagenesis, and neutralizing antibody approaches.
Results: rSjCa8 exhibited a striking inhibitory effect on macrophage migration, but did not markedly increase cell proliferation or apoptosis. Additionally, rSjCa8 potently inhibited NO release by LPS-stimulated macrophages in a dose- and time-dependent manner, and the inhibitory mechanism was closely associated with intracellular Ca(2+) levels, the up-regulation of catalase expression, and the down-regulation of the expression of 47 genes, including Myc, Gadd45a, Txnip, Fas, Sod2, Nos2, and Hmgb1. Vaccination with rSjCa8 increased NO concentration in the challenging skin area of infected mice and reduced the number of migrated schistosomula after skin penetration by cercariae.
Conclusions: Our findings indicate that SjCa8 might be a novel molecule that plays a critical role in immune evasion by S. japonicum cercaria during the process of skin penetration. The inhibitory impacts of rSjCa8 on macrophage migration and [Ca(2+)]i-dependent NO release suggest it might represent a novel vaccine candidate and chemotherapeutic target for the prevention and treatment of schistosomiasis.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

