Regulated Dynamic Trafficking of Neurexins Inside and Outside of Synaptic Terminals
- PMID: 26446217
- PMCID: PMC6605384
- DOI: 10.1523/JNEUROSCI.4041-14.2015
Regulated Dynamic Trafficking of Neurexins Inside and Outside of Synaptic Terminals
Abstract
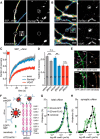
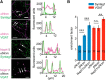
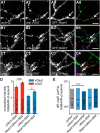
Synapses depend on trafficking of key membrane proteins by lateral diffusion from surface populations and by exocytosis from intracellular pools. The cell adhesion molecule neurexin (Nrxn) plays essential roles in synapses, but the dynamics and regulation of its trafficking are unknown. Here, we performed single-particle tracking and live imaging of transfected, epitope-tagged Nrxn variants in cultured rat and mouse wild-type or knock-out neurons. We observed that structurally larger αNrxn molecules are more mobile in the plasma membrane than smaller βNrxns because αNrxns displayed higher diffusion coefficients in extrasynaptic regions and excitatory or inhibitory terminals. We found that well characterized interactions with extracellular binding partners regulate the surface mobility of Nrxns. Binding to neurexophilin-1 (Nxph1) reduced the surface diffusion of αNrxns when both molecules were coexpressed. Conversely, impeding other interactions by insertion of splice sequence #4 or removal of extracellular Ca(2+) augmented the mobility of αNrxns and βNrxns. We also determined that fast axonal transport delivers Nrxns to the neuronal surface because Nrxns comigrate as cargo on synaptic vesicle protein transport vesicles (STVs). Unlike surface mobility, intracellular transport of βNrxn(+) STVs was faster than that of αNrxns, but both depended on the microtubule motor protein KIF1A and neuronal activity regulated the velocity. Large spontaneous fusion of Nrxn(+) STVs occurred simultaneously with synaptophysin on axonal membranes mostly outside of active presynaptic terminals. Surface Nrxns enriched at synaptic terminals where αNrxns and Nxph1/αNrxns recruited GABAAR subunits. Therefore, our results identify regulated dynamic trafficking as an important property of Nrxns that corroborates their function at synapses.
Significance statement: Synapses mediate most functions in our brains and depend on the precise and timely delivery of key molecules throughout life. Neurexins (Nrxns) are essential synaptic cell adhesion molecules that are involved in synaptic transmission and differentiation of synaptic contacts. In addition, Nrxns have been linked to neuropsychiatric diseases such as autism. Because little is known about the dynamic aspects of trafficking of neurexins to synapses, we investigated this important question using single-molecule tracking and time-lapse imaging. We identify distinct differences between major Nrxn variants both in surface mobility and during intracellular transport. Because their dynamic behavior is highly regulated, for example, by different binding activities, these processes have immediate consequences for the function of Nrxns at synapses.
Keywords: GABA(A) receptors; autism; imaging; neuroligin; quantum dots; synapse function.
Copyright © 2015 the authors 0270-6474/15/3513630-19$15.00/0.
Figures







References
-
- Beglopoulos V, Montag-Sallaz M, Rohlmann A, Piechotta K, Ahmad M, Montag D, Missler M. Neurexophilin 3 is highly localized in cortical and cerebellar regions and is functionally important for sensorimotor gating and motor coordination. Mol Cell Biol. 2005;25:7278–7288. doi: 10.1128/MCB.25.16.7278-7288.2005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
