Single-Agent Panitumumab in Frail Elderly Patients With Advanced RAS and BRAF Wild-Type Colorectal Cancer: Challenging Drug Label to Light Up New Hope
- PMID: 26446234
- PMCID: PMC4718427
- DOI: 10.1634/theoncologist.2015-0171
Single-Agent Panitumumab in Frail Elderly Patients With Advanced RAS and BRAF Wild-Type Colorectal Cancer: Challenging Drug Label to Light Up New Hope
Abstract
Background: No prospective trials have specifically addressed the efficacy and safety of panitumumab in elderly patients with metastatic colorectal cancer (CRC). We aimed at assessing the efficacy and safety of single agent panitumumab in "frail" elderly patients diagnosed with metastatic RAS and BRAF wild-type CRC.
Materials and methods: Forty elderly patients (aged ≥ 75 years) with metastatic RAS-BRAF wild-type CRC received off-label prescriptions of single-agent panitumumab at seven Italian institutions. Treatment was administered as first line in patients with absolute contraindication to any chemotherapy or as second-line treatment after failure of a fluoropyrimidine-based treatment, in the presence of contraindication to irinotecan. The outcome measures included objective response rate (ORR), as well as progression-free survival (PFS), disease control rate (DCR), overall survival (OS), and safety.
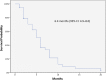
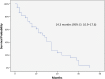
Results: The median PFS and OS were 6.4 months (95% confidence interval [CI]: 4.9-8 months) and 14.3 months (95% CI: 10.9-17.7 months), respectively. ORR was 32.5%, and DCR was 72.5%. Dose reductions related to adverse events (AEs) were reported in 9 (23%) patients, but no permanent treatment discontinuation caused by was reported. The most frequent grade 3 AE was skin rash, with an incidence of 20%.
Conclusion: Panitumumab is effective and well-tolerated in frail elderly patients with RAS-BRAF wild-type metastatic CRC and deemed unfit for chemotherapy. A randomized study is needed to confirm these data.
Implications for practice: Treatment of elderly patients with metastatic colorectal cancer represents a difficult challenge in clinical practice. A significant proportion of frail elderly patients do not receive treatment, reflecting ongoing uncertainty of clinical benefit and toxicity of chemotherapy. Unfit condition in this cohort of patients further limits antineoplastic prescription and consequently patient survival. RAS and BRAF wild-type status could help select an elderly and unfit population that could benefit from anti-epidermal growth factor receptor single agent therapy. In the present study, single-agent off-label panitumumab was effective and well-tolerated as first-line treatment in frail elderly patients deemed unfit for chemotherapy for metastatic RAS and BRAF wild-type colorectal cancer.
Keywords: Elderly; Frail; Metastatic colorectal cancer; Panitumumab.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. - PubMed
-
- Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. - PubMed
-
- Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: The PRIME study. J Clin Oncol. 2010;28:4697–4705. - PubMed
-
- De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: A retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous