Bispecific Antibodies Targeting Different Epitopes on the HIV-1 Envelope Exhibit Broad and Potent Neutralization
- PMID: 26446600
- PMCID: PMC4665248
- DOI: 10.1128/JVI.02097-15
Bispecific Antibodies Targeting Different Epitopes on the HIV-1 Envelope Exhibit Broad and Potent Neutralization
Abstract
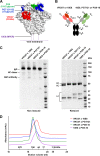
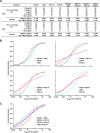
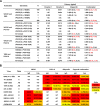
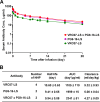
The potency and breadth of the recently isolated neutralizing human monoclonal antibodies to HIV-1 have stimulated interest in their use to prevent or to treat HIV-1 infection. Due to the antigenically diverse nature of the HIV-1 envelope (Env), no single antibody is highly active against all viral strains. While the physical combination of two broadly neutralizing antibodies (bNAbs) can improve coverage against the majority of viruses, the clinical-grade manufacturing and testing of two independent antibody products are time and resource intensive. In this study, we constructed bispecific immunoglobulins (IgGs) composed of independent antigen-binding fragments with a common Fc region. We developed four different bispecific IgG variants that included antibodies targeting four major sites of HIV-1 neutralization. We show that these bispecific IgGs display features of both antibody specificities and, in some cases, display improved coverage over the individual parental antibodies. All four bispecific IgGs neutralized 94% to 97% of antigenically diverse viruses in a panel of 206 HIV-1 strains. Among the bispecific IgGs tested, VRC07 × PG9-16 displayed the most favorable neutralization profile. It was superior in breadth to either of the individual antibodies, neutralizing 97% of viruses with a median 50% inhibitory concentration (IC50) of 0.055 μg/ml. This bispecific IgG also demonstrated in vivo pharmacokinetic parameters comparable to those of the parental bNAbs when administered to rhesus macaques. These results suggest that IgG-based bispecific antibodies are promising candidates for the prevention and treatment of HIV-1 infection in humans.
Importance: To prevent or treat HIV-1 infection, antibodies must potently neutralize nearly all strains of HIV-1. Thus, the physical combination of two or more antibodies may be needed to broaden neutralization coverage and diminish the possibility of viral resistance. A bispecific antibody that has two different antibody binding arms could potentially display neutralization characteristics better than those of any single parental antibody. Here we show that bispecific antibodies contain the binding specificities of the two parental antibodies and that a single bispecific antibody can neutralize 97% of viral strains with a high overall potency. These findings support the use of bispecific antibodies for the prevention or treatment of HIV-1 infection.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Pancera M, Zhou T, Druz A, Georgiev IS, Soto C, Gorman J, Huang J, Acharya P, Chuang GY, Ofek G, Stewart-Jones GB, Stuckey J, Bailer RT, Joyce MG, Louder MK, Tumba N, Yang Y, Zhang B, Cohen MS, Haynes BF, Mascola JR, Morris L, Munro JB, Blanchard SC, Mothes W, Connors M, Kwong PD. 2014. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature 514:455–461. doi:10.1038/nature13808. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

