Cell type-dependent mechanisms for formin-mediated assembly of filopodia
- PMID: 26446836
- PMCID: PMC4678021
- DOI: 10.1091/mbc.E15-09-0626
Cell type-dependent mechanisms for formin-mediated assembly of filopodia
Abstract
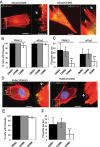
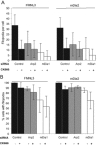
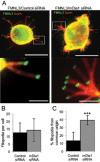
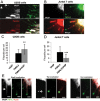
Filopodia are finger-like protrusions from the plasma membrane and are of fundamental importance to cellular physiology, but the mechanisms governing their assembly are still in question. One model, called convergent elongation, proposes that filopodia arise from Arp2/3 complex-nucleated dendritic actin networks, with factors such as formins elongating these filaments into filopodia. We test this model using constitutively active constructs of two formins, FMNL3 and mDia2. Surprisingly, filopodial assembly requirements differ between suspension and adherent cells. In suspension cells, Arp2/3 complex is required for filopodial assembly through either formin. In contrast, a subset of filopodia remains after Arp2/3 complex inhibition in adherent cells. In adherent cells only, mDia1 and VASP also contribute to filopodial assembly, and filopodia are disproportionately associated with focal adhesions. We propose an extension of the existing models for filopodial assembly in which any cluster of actin filament barbed ends in proximity to the plasma membrane, either Arp2/3 complex dependent or independent, can initiate filopodial assembly by specific formins.
© 2015 Young, Heimsath, and Higgs. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures




References
-
- Bachmann C, Fischer L, Walter U, Reinhard M. The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding, and actin bundle formation. J Biol Chem. 1999;274:23549–23557. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

