Rapid formation of a stable boron-nitrogen heterocycle in dilute, neutral aqueous solution for bioorthogonal coupling reactions
- PMID: 26446871
- PMCID: PMC4651731
- DOI: 10.1039/c5cc07453c
Rapid formation of a stable boron-nitrogen heterocycle in dilute, neutral aqueous solution for bioorthogonal coupling reactions
Abstract
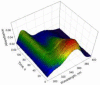
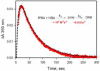
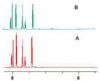
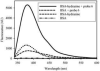
Combining 2-formylphenylboronic acid with 4-hydrazinylbenzoic acid in neutral aqueous solution at low, equimolar concentrations of the reagents results in a single, stable product, a 1,2-dihydro-1-hydroxy-2,3,1-benzodiazaborine, in a matter of minutes with no side products. Application of this reaction to protein conjugation demonstrates that the reaction is orthogonal to protein functional groups, and the resulting conjugate withstands SDS-PAGE analysis. This reaction should be particularly useful for couplings that must be performed with low concentrations of reagents under physiologically compatible conditions.
Figures
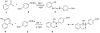
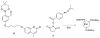

Similar articles
-
Boron enabled bioconjugation chemistries.Chem Soc Rev. 2024 Dec 9;53(24):11888-11907. doi: 10.1039/d4cs00750f. Chem Soc Rev. 2024. PMID: 39479937 Free PMC article. Review.
-
Novel synthesis of cinnolines and 1-aminoindolines via Cu-catalyzed intramolecular N-arylation of hydrazines and hydrazones prepared from 3-haloaryl-3-hydroxy-2-diazopropanoates.J Org Chem. 2008 Aug 15;73(16):6363-8. doi: 10.1021/jo8010864. Epub 2008 Jul 9. J Org Chem. 2008. PMID: 18610983
-
β-Hydroxy-Stabilized Boron-Nitrogen Heterocycles Enable Rapid and Efficient C-Terminal Protein Modification.Bioconjug Chem. 2019 Oct 16;30(10):2604-2613. doi: 10.1021/acs.bioconjchem.9b00534. Epub 2019 Sep 18. Bioconjug Chem. 2019. PMID: 31483610 Free PMC article.
-
Formation of hydrazones and stabilized boron-nitrogen heterocycles in aqueous solution from carbohydrazides and ortho-formylphenylboronic acids.Org Biomol Chem. 2017 Sep 20;15(36):7543-7548. doi: 10.1039/c7ob01708a. Org Biomol Chem. 2017. PMID: 28853481 Free PMC article.
-
Boronic Acids as Bioorthogonal Probes for Site-Selective Labeling of Proteins.Angew Chem Int Ed Engl. 2018 Oct 1;57(40):13028-13044. doi: 10.1002/anie.201712611. Epub 2018 Aug 23. Angew Chem Int Ed Engl. 2018. PMID: 29723444 Review.
Cited by
-
Fast Diazaborine Formation of Semicarbazide Enables Facile Labeling of Bacterial Pathogens.J Am Chem Soc. 2017 Jan 18;139(2):871-878. doi: 10.1021/jacs.6b11115. Epub 2017 Jan 3. J Am Chem Soc. 2017. PMID: 27992180 Free PMC article.
-
Boron enabled bioconjugation chemistries.Chem Soc Rev. 2024 Dec 9;53(24):11888-11907. doi: 10.1039/d4cs00750f. Chem Soc Rev. 2024. PMID: 39479937 Free PMC article. Review.
-
Site-Specific Bioconjugation and Multi-Bioorthogonal Labeling via Rapid Formation of a Boron-Nitrogen Heterocycle.Bioconjug Chem. 2019 May 15;30(5):1554-1564. doi: 10.1021/acs.bioconjchem.9b00246. Epub 2019 May 3. Bioconjug Chem. 2019. PMID: 31026151 Free PMC article.
-
The linkage-type and the exchange molecule affect the protein-labeling efficiency of iminoboronate probes.Org Biomol Chem. 2023 Nov 29;21(46):9173-9181. doi: 10.1039/d3ob01269g. Org Biomol Chem. 2023. PMID: 37947354 Free PMC article.
-
Boronic acid based dynamic click chemistry: recent advances and emergent applications.Chem Sci. 2020 Dec 17;12(5):1585-1599. doi: 10.1039/d0sc05009a. Chem Sci. 2020. PMID: 34163920 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

