Rapid formation of a stable boron-nitrogen heterocycle in dilute, neutral aqueous solution for bioorthogonal coupling reactions
- PMID: 26446871
- PMCID: PMC4651731
- DOI: 10.1039/c5cc07453c
Rapid formation of a stable boron-nitrogen heterocycle in dilute, neutral aqueous solution for bioorthogonal coupling reactions
Abstract
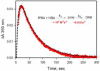
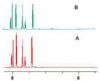
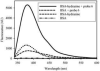
Combining 2-formylphenylboronic acid with 4-hydrazinylbenzoic acid in neutral aqueous solution at low, equimolar concentrations of the reagents results in a single, stable product, a 1,2-dihydro-1-hydroxy-2,3,1-benzodiazaborine, in a matter of minutes with no side products. Application of this reaction to protein conjugation demonstrates that the reaction is orthogonal to protein functional groups, and the resulting conjugate withstands SDS-PAGE analysis. This reaction should be particularly useful for couplings that must be performed with low concentrations of reagents under physiologically compatible conditions.
Figures
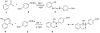
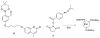

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

