Influence of companion diagnostics on efficacy and safety of targeted anti-cancer drugs: systematic review and meta-analyses
- PMID: 26446908
- PMCID: PMC4741844
- DOI: 10.18632/oncotarget.5946
Influence of companion diagnostics on efficacy and safety of targeted anti-cancer drugs: systematic review and meta-analyses
Abstract
Background: Companion diagnostics aim to identify patients that will respond to targeted therapies, therefore increasing the clinical efficacy of such drugs. Less is known about their influence on safety and tolerability of targeted anti-cancer agents.
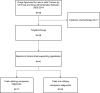
Methods and findings: Randomized trials evaluating targeted agents for solid tumors approved by the US Food and Drug Administration since year 2000 were assessed. Odds ratios (OR) and and 95% confidence intervals (CI) were computed for treatment-related death, treatment-discontinuation related to toxicity and occurrence of any grade 3/4 adverse events (AEs). The 12 most commonly reported individual AEs were also explored. ORs were pooled in a meta-analysis. Analysis comprised 41 trials evaluating 28 targeted agents. Seventeen trials (41%) utilized companion diagnostics. Compared to control groups, targeted drugs in experimental arms were associated with increased odds of treatment discontinuation, grade 3/4 AEs, and toxic death irrespective of whether they utilized companion diagnostics or not. Compared to drugs without available companion diagnostics, agents with companion diagnostics had a lower magnitude of increased odds of treatment discontinuation (OR = 1.12 vs. 1.65, p < 0.001) and grade 3/4 AEs (OR = 1.09 vs. 2.10, p < 0.001), but no difference in risk of toxic death (OR = 1.40 vs. 1.27, p = 0.69). Differences between agents with and without companion diagnostics were greatest for diarrhea (OR = 1.29 vs. 2.43, p < 0.001), vomiting (OR = 0.86 vs. 1.44, p = 0.005), cutaneous toxicity (OR = 1.82 vs. 3.88, p < 0.001) and neuropathy (OR = 0.64 vs. 1.60, p < 0.001).
Conclusions: Targeted drugs with companion diagnostics are associated with improved safety, and tolerability. Differences were most marked for gastrointestinal, cutaneous and neurological toxicity.
Keywords: cancer drugs; companion diagnostics; efficacy; trial design.
Conflict of interest statement
No authors have any competing interests. An ethics statement was not required for this work. No funding was received for this work.
Figures
References
-
- Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, Silliman N, Szabo S, Dezso Z, Ustyanksky V, Nikolskaya T, Nikolsky Y, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. - PubMed
-
- Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, Varella-Garcia M, Franklin WA, Aronson SL, Su PF, Shyr Y, Camidge DR, Sequist LV, Glisson BS, Khuri FR, Garon EB, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. Jama. 2014;311:1998–2006. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources